PARIS--(BUSINESS WIRE)--Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported that Leber Hereditary Optical Neuropathy (LHON) subjects treated with LUMEVOQ® continued to experience significantly improved vision four years after a single injection of the gene therapy. The findings come from RESTORE (CLIN06), the long-term follow-up study to which participants in the RESCUE1 and REVERSE2 Phase III pivotal trials were invited.
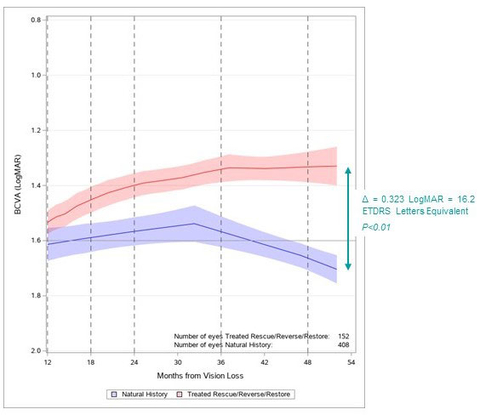
When RESTORE subjects enrolled in the study, 2 years after the one-time injection, they had already experienced clinically meaningful improvements relative to the lowest point (the “nadir”) of their best-corrected visual acuity (BCVA): +18.8 ETDRS letters equivalent* in their LUMEVOQ®-treated eyes and +17.3 letters equivalent in their sham-treated eyes. Four years after treatment, the bilateral improvement from nadir was sustained, with LUMEVOQ®-treated eyes achieving a mean improvement against nadir of +22.5 letters equivalent and sham-treated eyes demonstrating a mean improvement of +20.5 letters equivalent.
The impact of such results on patients is demonstrated by increases in the self-reported quality of life (QoL) scores at Year 4 vs. baseline. Mean overall QoL increased by a clinically meaningful magnitude relative to baseline, driven by clinically meaningful increases in the sub-scores corresponding to mental health and the ability to carry out activities autonomously (e.g., role difficulties, dependency, near and far activities, general vision).
“The 4-year RESTORE long-term extension study provides patients with Leber Hereditary Optic Neuropathy and their families as well as the neuro-ophthalmology community with highly informative data about both the efficacy and safety of intravitreal LUMEVOQ therapy,” commented Dr. Robert Sergott, Director, Neuro-Ophthalmology Service, Wills Eye Hospital, and Founding Director and CEO, William H. Annesley EyeBrain Center, Thomas Jefferson University, Philadelphia, PA, USA. “Compared to the natural history of LHON, the 4-year data extend and validate the 3-year observations by confirming that objective visual acuity improvement is sustained and is associated with improved functional visual quality of life without any long-term safety concern.”
“The RESTORE findings underline the therapeutic value of GenSight’s pioneering one-time treatment for LHON: durable and clinically significant improvement in visual function coupled with impressive safety,” noted Bernard Gilly, Co-founder and Chief Executive Officer of GenSight. “The body of evidence we have now accumulated is without doubt good news for patients needing an urgent solution for their brutal blinding condition, and consequently we are continuing to work vigorously with the relevant authorities to bring regulatory review process to a successful conclusion.”
RESTORE is one of the largest long-term follow-up studies for a rare disease treatment, with 62 subjects accepting the invitation to enroll. All subjects, who were affected by LHON caused by a mutated ND4 mitochondrial gene, were treated with an intravitreal injection of LUMEVOQ® in one eye and with sham injection in the other.
Table 1. BCVA Mean Improvement Vs. Nadir* In LUMEVOQ® Long-Term Follow-Up (RESTORE)
|
2 Years Post-Injection |
3 Years Post-Injection3 |
4 Years Post-Injection |
|||
LogMAR
|
Letters
|
LogMAR
|
Letters
|
LogMAR
|
Letters
|
|
LUMEVOQ®-treated eyes |
-0.375
|
+18.8 |
-0.410
|
+20.5 |
-0.453
|
+22.5 |
Sham-treated eyes |
-0.346
|
+17.3 |
-0.387
|
+19.4 |
-0.406
|
+20.5 |
Note: The RESTORE sample consists of the RESCUE and REVERSE participants who accepted to be followed in the long-term follow-up study. Year 4 values were the LogMAR readings nearest to 1461 days post treatment recorded between 1461 +/- 273 days post- treatment. Missing values were imputed using the Last Observation Carried Forward (LOCF) method. *Nadir = worst best-corrected visual acuity recorded from baseline to Year 4. ** Assessments of best-corrected visual acuity (BCVA) were recorded in LogMAR. The change from nadir in LogMAR was converted to “letters equivalent” improvement by multiplying the LogMAR by -50 (ref. J.T. Holladay, J Refrac Surgery, 1997;13, 388-391).
Responder analyses at Year 4 indicate that improved BCVA was a benefit for a substantial proportion of the study participants. 71.0% of RESTORE subjects achieved Clinically Relevant Recovery (CRR)4 against nadir four years after treatment, and 80.7% of them had on-chart vision (BCVA ≤ 1.6 LogMAR) in one or both eyes.
Viewed against the trend in vision typically seen in untreated patients5, the findings represent a significant departure from the natural progression of LHON.
Safety findings at 4 years post-injection were consistent with previous readouts, which concluded that LUMEVOQ® is well-tolerated: no serious adverse events were recorded among LUMEVOQ®-treated eyes, and no discontinuations occurred due to ocular events. There were no systemic serious adverse events or discontinuations related to study treatment or study procedure.
The review of the European Marketing Authorisation Application for LUMEVOQ® is ongoing, with the decision from the CHMP expected in Q4 2022.
References and notes:
- Newman NJ, Yu-Wai-Man P, Carelli V, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology (2021) 128:649–60. doi: 10.1016/j.ophtha.2020.12.012.
- Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. (2020) 12:eaaz7423. doi: 10.1126/scitranslmed.aaz7423.
- Biousse, V, Newman, N, Yu-Wai-Man P, et al. Long-Term Follow-Up After Unilateral Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy: The RESTORE Study, J Neuroophthalmol. (2021) 41: 309-315. doi: 10.1097/WNO.0000000000001367.
- Clinically Relevant Recovery (CRR) corresponds to an improvement of at least 0.2 LogMAR (for on-chart eyes) or a movement from off-chart to on-chart (for off-chart eyes).
- Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual outcomes in Leber hereditary optic neuropathy subjects with the m.11778G>A (MTND4) mitochondrial DNA mutation. J Neuroophthalmol. (2020) 40:547–57. doi: 10.1097/WNO.0000000000001045.
- Newman NJ, Yu-Wai-Man P, Carelli V, et al.,. Intravitreal Gene Therapy vs. Natural History in Patients With Leber Hereditary Optic Neuropathy Carrying the m.11778G>A ND4 Mutation: Systematic Review and Indirect Comparison. Front. Neurol. (2021) 12:662838. doi: 10.3389/fneur.2021.662838.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of subjects have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,200-1,500 new subjects who lose their sight every year in the United States and the European Union.
About LUMEVOQ® (GS010; lenadogene nolparvovec)
LUMEVOQ® (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. “LUMEVOQ” was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018.
About RESCUE, REVERSE, and RESTORE
RESCUE and REVERSE were two separate randomized, double-masked, sham-controlled Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint measured the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best‑Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, were used for statistical purposes. Both trials were adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between drug-treated and sham-treated eyes, adjusted to baseline.
The secondary endpoints involved the application of the primary analysis to best‑seeing eyes that received GS010 compared to those receiving sham, and to worse‑seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis was performed, including the proportion of patients who maintained vision (< ETDRS 15L loss), the proportion of patients who gained 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics included automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality-of-life scales, bio‑dissemination and the time course of immune response. Readouts for these endpoints were at 48, 72 and 96 weeks after injection.
The trials were conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Week 96 results were reported in 2019 for both trials, after which patients were invited to participate in a long-term follow-up study, RESTORE, for three additional years.
The primary objective is to assess the long-term safety of intravitreal LUMEVOQ® administration up to 5 years post-treatment. The secondary objective is to assess the long-term treatment efficacy of the therapy and the quality of life (QoL) in subjects up to 5 years post-treatment. The first subject was enrolled on January 9, 2018. 61 subjects have enrolled.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767
RESTORE: NCT03406104