Lazurite™ Announces FDA 510(k) Submission for its ArthroFree™ Wireless Camera System; is Recognized by AngelMD as One of the Best Startups of 2021
Lazurite™ Announces FDA 510(k) Submission for its ArthroFree™ Wireless Camera System; is Recognized by AngelMD as One of the Best Startups of 2021
CLEVELAND--(BUSINESS WIRE)--Medical device and technology company Lazurite Holdings LLC today announced it has submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for its ArthroFree™ wireless camera system for minimally invasive surgery, and that the submission has been accepted for review. The company also announced that early-stage healthcare investment platform AngelMD named it one of the best startup companies of 2021.
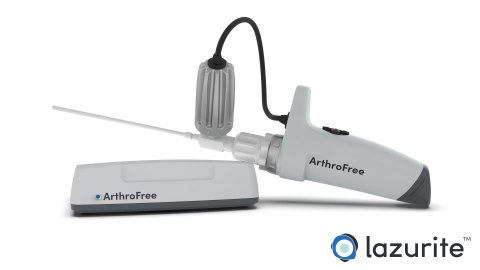
The ArthroFree system is expected to be the world’s first FDA-approved fully wireless, minimally invasive camera system for the operating room. The modular system incorporates the company’s proprietary low-heat, high-intensity Meridiem™ light engine technology along with advanced camera, battery, and wireless transmission technologies. The ArthroFree system is designed to deliver improved operating room productivity, patient safety, and economic value through cost-savings, energy efficiency, and reduced setup/breakdown times. The system is designed to be fully drop-in compatible with current operating room technology.
“This FDA submission marks an important milestone in our commitment to bring an advanced wireless surgical camera to market," said Eugene Malinskiy, chief executive officer and co-founder of Lazurite. “During the nearly 50 years since the introduction of the first fiber-optic scope, the industry has seen only a slow evolution in image quality, monitors, and usability. The ArthroFree system is designed to untether the camera from the surgical tower, which is expected to make it safer and easier to use, while reducing OR setup and turnover times and delivering exceptional light and image quality during minimally invasive surgery.”
“The ArthroFree system promises to usher in a new era of wireless, minimally invasive camera systems for the operating room,” said Mark Froimson, MD, Chair of the Board of Managers of Lazurite. “Our FDA approval process doesn’t require any human or animal trials, so there is good reason to believe that we will receive FDA clearance for market launch by mid-2022. Based on the response from surgeons we’ve met at industry trade shows and on visits to leading medical centers across the country, we believe the system will be poised to redefine industry expectations for the use of surgical cameras.”
AngelMD recognition
AngelMD is an online investment platform and expert community connecting thousands of clinicians, startups, investors, and other healthcare industry stakeholders with interest in helping to evaluate, advise and invest in promising startups.
AngelMD wrapped up 2021 with a “Best Of” startup competition in three categories: Cardiology, Orthopedics and General. After an initial nomination process, the competition ran two weeks and had hundreds of participants vote for their favorite startup. Lazurite was one of three companies recognized in the Orthopedics category.
“We host Pitch Clubs, Impact Summits and competitions like this to help create visibility for very deserving startups and entrepreneurs. While there were lots of great startups in the nomination pool, these companies are clearly deserving of the recognition,” said Katie Richardson, MD, AngelMD.
“It is especially gratifying to be recognized by the AngelMD community,” said Malinskiy. “These healthcare professionals are dedicated to finding and supporting startups with the potential to benefit their practices, their patients’ well-being, and even the future of healthcare. We are proud to have some of them among our investors.”
This was the fourth such national award Lazurite received in 2021. This past summer, the ArthroFree system won the prestigious inaugural ACE Award for Innovation at the annual meeting of the American Orthopaedic Society for Sports Medicine and was one of three finalists in the Shark Tank competition at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons. In December, MedTech Outlook magazine named Lazurite a top 10 endoscopy device provider for 2021.
The ArthroFree™ wireless surgical camera system has not yet received FDA clearance and is not currently approved for human use. It is not intended for commercial distribution; orders cannot be accepted at this time.
About Lazurite
Lazurite (formerly Indago) is a pre-revenue medical device startup company backed by private capital. The company has raised more than $18 million to date from institutional investors, family offices, and more than 50 physician champions. Founded in Cleveland in 2015, Lazurite has developed novel technology that will enable it to create the operating room of the future. The company’s product pipeline features the modular ArthroFree™ wireless camera system, which is expected to be the first FDA-approved fully wireless minimally invasive camera system platform designed for the operating room, and other innovative new products based on the company’s novel and patented Meridiem™ light engine technology. Lazurite’s technologies are protected by a comprehensive intellectual property portfolio encompassing our ArthroFree wireless surgical camera system and our novel Meridiem light source as well as other products currently in development. For more information, see: https://lazurite.co.
Forward Looking Statements
This press release includes “forward-looking statements." Forward-looking statements can be identified by words such as: “anticipate,” “intend,” “plan,” “believe,” “project,” “estimate,” “expect,” “may,” “should,” “will,” “designed,” “milestone,” and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding our regulatory submission and clearance timelines for ArthroFree™. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations, and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy, and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Any forward-looking statement made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Lazurite™, ArthroFree™, and Meridiem™ are trademarks of Lazurite Holdings LLC.
Contacts
Pat Gallagher
Patrick Gallagher PR & Marketing Communications
pat@PatGallagherPR.com
+1 (216) 233-7473
Lazurite: +1 (833) 214-2324
contact@lazurite.co