Allurion Technologies Announces New Data on the Impact of the Allurion Program on Type 2 Diabetes and Prediabetes
Allurion Technologies Announces New Data on the Impact of the Allurion Program on Type 2 Diabetes and Prediabetes
Program leads to remission of Type 2 Diabetes with reduction in average hemoglobin A1c of 1.5% combined with average Total Body Weight Loss of 16.2% after just 4 months
NATICK, Mass.--(BUSINESS WIRE)--Allurion Technologies, a company dedicated to ending obesity, today announced new clinical data on the Allurion Program presented at ObesityWeek® 2021. The Allurion Program is a 360-degree weight loss experience featuring the Allurion Balloon, the world’s first and only swallowable, procedureless gastric balloon for weight loss, remote patient monitoring through the Allurion Virtual Care Suite including the Allurion Connected Scale, Health Tracker, and App, and Allurion Insights, a provider portal that enables message, telehealth, and end-to-end patient management.
Despite the availability of pharmacologic therapies for Type 2 Diabetes, the incidence of metabolic disease continues to rise around the world. According to the International Diabetes Federation, 537 million adults are now living with diabetes worldwide, an increase of 16% (74 million people) since 2019. By 2045, 783 million people—or 1 in 8 adults—will be living with diabetes worldwide, indicating that current treatment options are not sufficient.
“Obesity and Type 2 Diabetes are pandemics of unprecedented magnitude, and they often afflict patients simultaneously,” said Dr. Shantanu Gaur, co-founder and CEO of Allurion. “Current treatments for obesity and diabetes either suffer from poor safety and efficacy or a poor patient experience. The Allurion Program offers a compelling alternative for the treatment of both of these conditions.”
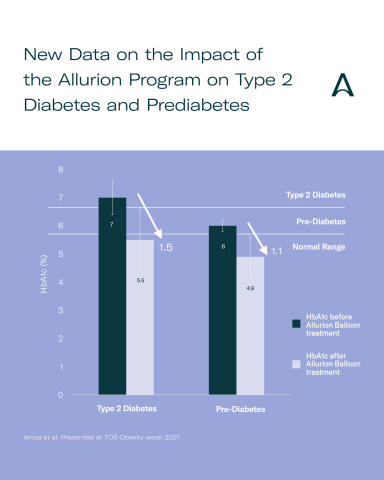
In an abstract submitted and accepted for presentation at ObesityWeek 2021 titled Allurion Gastric Balloon Program is an Emerging Novel Treatment for Type 2 Diabetes and Prediabetes, Dr. Roberta Ienca and colleagues report on 226 patients (78 with Type 2 Diabetes and 148 with prediabetes) who enrolled in the Allurion Program and were followed for a period of 4 months. After 4 months, mean hemoglobin A1c (HbA1c) decreased from 7.0% to 5.5% with a mean weight loss of 19.3kg in patients with diabetes. Remission of diabetes is typically defined as lowering HbA1c to below 6.5%, indicating that the average patient in the diabetes group achieved remission after just 4 months. In patients with prediabetes, mean HbA1c decreased from 6.0% to 4.9% with a mean weight loss of 16.9kg.
“The Allurion Program provides patients with an effective tool to reduce HbA1c and put Type 2 Diabetes into remission,” said Dr. Ram Chuttani, Chief Medical Officer of Allurion. “These results underscore the benefits of the Allurion Program beyond weight loss and highlight how quickly the Allurion Program can have an impact on patients with Type 2 Diabetes or prediabetes.”
About Allurion Technologies
Allurion Technologies is dedicated to ending obesity. The Allurion Program is a 360-degree weight loss experience featuring the Allurion Gastric Balloon, the world’s first and only swallowable, procedureless gastric balloon for weight loss, the Allurion Virtual Care Suite including the Allurion Connected Scale, Health Tracker, and App, and Allurion Insights. Learn more about Allurion online at www.allurion.com. Allurion is a trademark of Allurion Technologies, Inc. in the United States and countries around the world.
Contacts
Whitney Cypes +1 (408)-547-7531