The Cystic Fibrosis Foundation Affirms Support of the Introduction of the PASTEUR Act
The Cystic Fibrosis Foundation Affirms Support of the Introduction of the PASTEUR Act
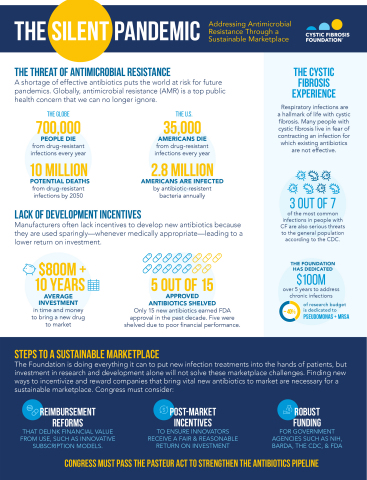
A shortage of effective antibiotics puts the world at risk for future pandemics. Globally, antimicrobial resistance (AMR) is a top public health concern that we can no longer ignore. Finding new ways to incentivize and reward companies that bring vital new antibiotics to market are necessary for a sustainable marketplace. (Graphic: Business Wire)
BETHESDA, Md.--(BUSINESS WIRE)--Today, the Cystic Fibrosis Foundation affirmed its support for the PASTEUR Act, a bipartisan proposal that, if passed, will support the development of new antibiotics and promote appropriate use of existing ones. The organization issued the following statement:
“New solutions are needed now to bring novel antibiotics into the hands of patients. The Cystic Fibrosis Foundation applauds Senators Bennet and Young in reintroducing the Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) Act. If passed, the PASTEUR Act has the potential to spur vital investment into new antibiotics by addressing the economic disincentives that have long been associated with antibiotic development.
“Routine use of antibiotics in cystic fibrosis care is medically necessary but unfortunately, too many people with CF find themselves battling difficult-to treat infections where existing antibiotics are not effective. However, antibiotic resistance is not solely a CF problem—it’s a human problem that we can no longer ignore.
“People with CF need more antibiotic options as do all Americans. The Foundation is making significant investments into the research and development of new approaches to address infections, but more is needed. Congress must take swift action to pass the PASTEUR Act, invigorating a new era of antibiotic research and development that could bring novel treatments into the hands of patients who need them the most.”
For more information about the Foundation’s work in fighting antimicrobial resistance, visit cff.org.
About the Cystic Fibrosis Foundation
The Cystic Fibrosis Foundation is the world's leader in the search for a cure for cystic fibrosis. The Foundation funds more CF research than any other organization, and nearly every CF drug available today was made possible because of Foundation support. Based in Bethesda, Md., the Foundation also supports and accredits a national care center network that has been recognized by the National Institutes of Health as a model of care for a chronic disease. The CF Foundation is a donor-supported nonprofit organization. For more information, visit cff.org.
Contacts
Ashley Mahoney
Email: amahoney@cff.org
Phone: 240-200-3754