First-in-Class GPR119 Agonist of Dong-A ST, DA-1241 Improved Glucose Control in Patients With Type 2 Diabetes in US Phase 1b Study
First-in-Class GPR119 Agonist of Dong-A ST, DA-1241 Improved Glucose Control in Patients With Type 2 Diabetes in US Phase 1b Study
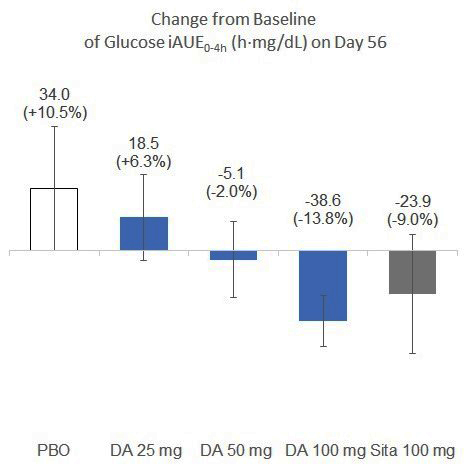
Ph1b Data Graph: Dong-A ST completed the proof-of-concept study for DA-1241, an investigational first-in-class small molecule with a mechanism of GPR119 agonist under clinical development, in patients with type 2 diabetes. In the US Phase 1b clinical study, 8-week treatment of ‘DA-1241’ 25mg, 50mg and 100mg showed the changes of +6.3%, -2.0% and -13.8% in iAUE levels from the baseline and DA-1241 100mg showed similar blood glucose improvement with that of Sitagliptin 100mg (-9.0%), and it outperformed placebo (+10.5%). (Graphic: Business Wire)
SEOUL, South Korea--(BUSINESS WIRE)--Dong-A ST (KRX: 170900) today announced that it successfully completed the proof-of-concept study for ‘DA-1241,’ an investigational first-in-class small molecule with a mechanism of GPR119 agonist under clinical development, in patients with type 2 diabetes.
In the US Phase 1b clinical study, 8-week treatment of DA-1241 in type-2 diabetic patients found an increase in clinical biomarkers by activating GPR119, and from a head-to-head comparison with Sitagliptin, a widely used dipeptidyl peptidase 4 (DPP4) inhibitor, in the study, DA-1241 showed glucose-lowering efficacy similar to Sitagliptin, which also indicates a higher clinical glucose control efficacy of DA-1241 compared to previous GPR119 agonists.
In the mid-2000s, GPR119 drew attention as a safe drug target for controlling glucose levels with no risk of hypoglycemia as it was reported to stimulate glucose-dependent insulin secretion in the pancreatic beta cells. Thus, many pharmaceutical companies in Korea and other countries participated in the competition of developing new GPR119 agonists. However, early-stage clinical studies of previous GPR119 agonists had released since 2011 showed disappointing results. These drugs showed insufficient efficacy or loss of efficacy after repeated administration and some raised the possibility of ethnic differences with less efficacy in the Western population compared to the Asian population.
Hence, after having selected DA-1241, a clinical candidate differentiated from previous GPR119 agonists based on non-clinical study results, Dong-A ST has been conducting clinical development of ‘DA-1241’ in the United States since early 2017. It conducted Phase 1a clinical study with healthy subjects (single ascending dose study) and then Phase 1b clinical study with both healthy subjects and type-2 diabetic patients (multiple ascending dose study).
The Phase 1b study was designed as a placebo and active comparator (Sitagliptin 100mg)-controlled, double-blinded and randomized study with an objective of evaluating whether ‘DA-1241’ delivers improved glucose-lowering efficacy in 83 diabetic patients. Patients were treated with placebo, Sitagliptin 100mg or ‘DA-1241’ 25mg, 50mg and 100mg once daily for 8 weeks, in combination with stable doses of metformin (13~19 patients/group). In the mixed meal tolerance test to evaluate the ability to reduce postprandial glucose through GPR119 activation, the incremental AUE0-4h of plasma glucose (iAUE) upon nutrient ingestion was measured and compared. 8-week treatment of ‘DA-1241’ 25mg, 50mg and 100mg showed the changes of +6.3%, -2.0% and -13.8% in iAUE levels from the baseline and DA-1241 100mg showed similar blood glucose improvement with that of Sitagliptin 100mg (-9.0%), and it outperformed placebo (+10.5%).
In the parameters of glycemic variability measured with a Continuous Glucose Monitoring (CGM) system and fasting plasma glucose, the glucose-lowering efficacy by ‘DA-1241’ was similar to that of Sitagliptin. Moreover, the time-in-range, the percentage of how long blood glucose value is within 70~180mg/dL, was increased by mitigating the hypoglycemia risk and duration of hyperglycemia whereas such time-in-range was reduced in the placebo group.
Single administration or 8-week repeated administration of DA-1241 increased secretion of gut peptide hormones such as glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP) and peptide YY (PYY) in gastrointestinal tracts after taking meals, while secretion of these hormones in Sitagliptin group was sequentially reduced, which verified the changes in biomarkers upon activating GPR119 by ‘DA-1241’. The amount of secretion of such hormones increased in proportion to the extent of exposure to ‘DA-1241’.
In terms of safety, no clinically significant adverse events were observed following the 8-week treatment, confirming the tolerability of DA-1241, and the bodyweight showed a tendency to decrease; Compared to placebo (-0.3%) and Sitagliptin (-0.3%), the weight loss rate was relatively high in DA-1241 100mg group with -2.2% (-1.57kg).
The results of this Phase 1b study are meaningful in that non-clinical differentiation of ‘DA-1241’ was translated in a clinical study. With the success of the Phase 1b clinical study of ‘DA-1241,’ Dong-A ST is preparing for a Phase 2 clinical study in diabetic patients, and two abstracts of top-line data for healthy subjects and type-2 diabetic patients respectively on the Phase 1b study have been submitted to the American Diabetes Association for presentation at its conference in June 2021.
GPR119
As an abbreviation of G protein-coupled receptor 119, GPR119 is the class A GPCR first cloned in the pancreas. It mediates the transmission of signal in cells as cyclic AMP is increased when the receptor is activated. It improves postprandial glucose by enhancing insulin secretion in pancreatic beta cells by glucose or lipid metabolites. GPR119 is also expressed in the small intestine in large amounts and it stimulates GLP-1 secretion, one of the incretin hormones, when it is activated by fatty acid metabolites. Secreted GLP-1 inhibits the final step of fat absorption by locally acting within the small intestine. And activation of GPR119 in the liver inhibits the biosynthesis of fatty acid. As the effect of dropping blood glucose levels and improving dyslipidemia was demonstrated in the early-stage clinical development, previous GPR119 agonists had drawn attention as a drug mechanism that improves blood glucose and lipid metabolism simultaneously. However, their clinical development was suspended because many large pharmaceutical companies failed in proving sufficient clinical efficacy in type-2 diabetic patients.
DA-1241
Having perceived the potentiality of GPR119 target, Dong-A ST discovered a clinical candidate, ‘DA-1241’ (GPR119 agonist) that has the effect of improving blood glucose through an in-house drug discovery program aiming to explore a substance with differentiated property from existing GPR119 agonists. In non-clinical animal studies, ‘DA-1241’ efficiently lowered both blood glucose and lipid levels simultaneously. After confirming differentiations of DA-1241 in the pre-clinical program, Dong-A ST conducted Phase 1a clinical study and Phase 1b clinical study, respectively in the United States. Building on the successful completion of Phase 1b clinical study of ‘DA-1241,’ Dong-A ST is preparing for Phase 2 clinical study to evaluate the efficacy for diabetes and dyslipidemia.
Contacts
Dong-A ST Co., Ltd
Jin-Seok Jeong, +82-2-920-8212
treadwheel@donga.co.kr
So-Ra Jo, +82-2-920-8224
sol@donga.co.kr