FDA Approves joimax® Intracs®em Electromagnetic Navigation System for Endoscopic Minimally Invasive Spine Surgery
FDA Approves joimax® Intracs®em Electromagnetic Navigation System for Endoscopic Minimally Invasive Spine Surgery
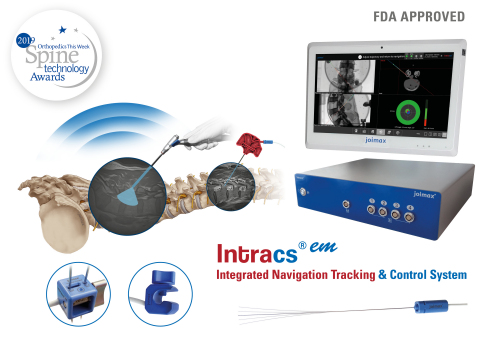
IRVINE, Calif.--(BUSINESS WIRE)--joimax®, the German-based market leader in technologies and training methods for full-endoscopic and minimally invasive spinal surgery, is pleased to announce that Intracs®em, its electromagnetic navigation tracking and control system, has been approved by the FDA.
The Intracs®em Navigation System allows for spinal procedures to be carried out with minimal X-ray control, drastically reducing radiation exposure, access time, and the entire length of the surgical procedure. In fact, all joimax® instruments and scopes can be tracked and accurately navigated through the appropriate sensors, which improves safety, expands endoscopic indications, and flattens the learning curve.
Intracs®em is a multiyear development. Its first cadaveric trials were made with a group of surgeons at the ESPINEA® Labs in Karlsruhe, Germany and Irvine, California.
According to Prof. Dr. med. Michael Kraus of ORTHIX Zentrum in Augsburg, Germany, who headed the preliminary trials: “Using Intracs®em Navigation System in the full endoscopic spine surgery is safe and easily applicable. The technology helps master precise access to the spine, and the clinical trials have confirmed the technique’s multiple advantages.”
Prof. Dr. Chris Hofstetter of UW Medical Center in Seattle, Washington, USA, adds: “joimax® Intracs®em constitutes a highly precise image-guided navigation platform designed for the specific needs of full-endoscopic spine surgery. Using electromagnetic navigation technology allows to track the leading edge of endoscopic instruments in real time, and facilitates needle targeting and orientation during the surgery, while minimizing radiation exposure. No doubt, this technology will expand the indications for full-endoscopic spine surgery and pave the way for innovations in minimally invasive arthrodesis techniques.”
joimax® is also excited to announce its second joint Global Symposium being held in partnership with Integrity Implants, Inc., August 21 - 22. For details, visit https://www.joimax.com/en/education-workshops/keep-in-touch-with-joimax/joimax-online-education-for-surgeons/
About joimax®
Founded in Karlsruhe, Germany in 2001, joimax® is the leading developer and marketer of complete systems for full-endoscopic and minimally invasive spinal surgery. With the Endoscopic Surgical Systems TESSYS® (transforaminal), iLESSYS® (interlaminar) and CESSYS® (cervical) for decompression procedures, MultiZYTE® for facet and sacroiliac joint pain treatment, EndoLIF® and Percusys® for minimally-invasive endoscopically assisted stabilizations, established systems are provided, addressing a whole range of indications. In procedures for herniated disc, stenosis, pain therapy or spinal stabilization treatment, surgeons utilize joimax® technologies to operate through small incisions under local or full anesthesia, via tissue and muscle-sparing corridors and through natural openings in the spinal canal, e.g. the intervertebral foramen, the so-called “Kambin triangle”.
Contacts
Press Contact Germany:
joimax® GmbH
Tatjana Elschnig
Tatjana.Elschnig@joimax.com
+49-721-25514-0
Press Contact USA:
joimax® Inc.
Emily Campbell
emily.campbell@joimaxusa.com
+1-949-859-3472