Sedative that Received FDA Emergency Use Authorization Now Available from Fresenius Kabi for use in Mechanically Ventilated COVID-19 patients
Sedative that Received FDA Emergency Use Authorization Now Available from Fresenius Kabi for use in Mechanically Ventilated COVID-19 patients
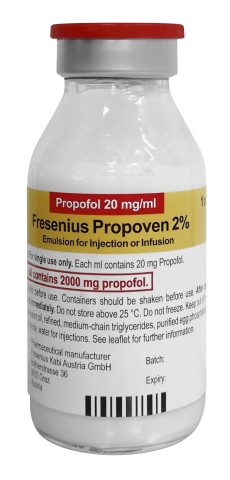
FDA authorization allows importation of Propoven 2% (propofol 20 mg per mL), double the concentration of the widely used drug Diprivan®
COVID-19 patients requiring mechanical ventilation increased demand for propofol worldwide
LAKE ZURICH, Ill.--(BUSINESS WIRE)--Fresenius Kabi announced today that following the recent U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA), Fresenius Kabi Propoven 2% (propofol 20 mg/mL) Emulsion 100 mL is now available in the U.S. The drug provides sedation via continuous infusion in patients greater than 16 years old who require mechanical ventilation in an intensive care unit (ICU).
Fresenius Kabi Propoven 2% Emulsion is not an FDA-approved drug in the United States, but it is approved in Europe and in other countries in a 50mL presentation. Propoven 2% in vials has been available since 2000 and is approved in 58 countries.
Fresenius Kabi Propoven 2% Emulsion contains the same active ingredient, propofol, as Fresenius Kabi’s FDA-approved Diprivan® Injectable Emulsion USP 10 mg/mL (diprivan-us.com) but contains double the concentration.
“Since the beginning of this pandemic, Fresenius Kabi has taken many steps to prioritize the production of products that are essential in helping to treat COVID-19,” said John Ducker, president and CEO of Fresenius Kabi USA. “We are grateful to the FDA for providing this Emergency Use Authorization for Propoven 2% as another solution to help severely ill patients during this public health crisis. We will continue to do all we can as a company, and in collaboration with others, to support caregivers and patients.”
“The availability in the U.S. of Fresenius Kabi Propoven 2% under Emergency Use Authorization provides clinicians with another sedation option for their COVID-19 patients,” said Seema Kumbhat, M.D., senior vice president and regional medical director at Fresenius Kabi USA. “A higher concentration presentation of propofol could be helpful to providers managing COVID-19 patients who require mechanical ventilation. However, since U.S. clinicians are not familiar with Fresenius Kabi Propoven 2%, we will be providing key information about the product’s differences from Diprivan 1% and making other product materials available to support the appropriate use of this product in the U.S.”
Fresenius Kabi will manage the distribution of this product through direct shipments and provide additional product-related materials including alert stickers to place directly on the vial, wall charts to display for use in areas such as hospital pharmacies and ICUs, a health care provider fact sheet and a patient and caregiver fact sheet.
The EUA of Fresenius Kabi Propoven 2% is supported by a Secretary of Health and Human Services declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic. The EUA for Fresenius Kabi Propoven 2% Emulsion is in effect for the duration of the COVID-19 declaration justifying emergency use of the products, unless terminated or revoked (after which the products may no longer be needed). The EUA will end when the declaration is terminated or revoked or when there is a change in the approval status of the product such that an EUA is no longer needed.
The scope of the EUA provides that Fresenius Kabi Propoven 2% Emulsion:
- Will be used only to maintain sedation via continuous infusion in patients greater than 16 years old who require mechanical ventilation.
- Will be administered only by a licensed health care provider in an ICU setting.
- Will not be administered to pregnant women, unless there are no FDA-approved products available to provide sedation for these patients should they require mechanical ventilation in an ICU.
- Will be used only in accordance with the dosing regimens as detailed below in the authorized Fact Sheets, which are available on Fresenius Kabi USA’s website (www.fresenius-kabi.com/us).
About Propoven 2%
Consistent with the EUA, Fresenius Kabi USA will offer the following presentation of Fresenius Kabi Propoven 2% (propofol 20 mg/mL) Emulsion:
Product Name and Description |
MCT/LCT Concentration |
Source/Type
|
Size |
Fresenius Kabi Propoven 2% Propofol Emulsion for Continuous Infusion 2,000 mg/100 mL (20 mg/mL) |
Medium Chain Triglycerides (MCT) 50 mg/mL Long Chain Triglycerides (LCT) 50 mg/mL |
soybean oil, refined; medium-chain triglycerides |
100 mL |
*NOTE: This propofol 20 mg per mL product contains double the concentration of propofol compared to the FDA-approved and marketed Diprivan® (propofol) Injectable Emulsion, USP 10 mg/mL product.
The Fresenius Kabi Propoven 2% Emulsion 100mL product will be manufactured by Fresenius Kabi AG in the same FDA-inspected facilities as Diprivan® and other Propoven 2% fill sizes.
ADVERSE EVENT REPORTING
Adverse events or quality problems experienced with the use of this product should be reported to the FDA or Fresenius Kabi. Health care facilities and prescribing health care providers or their designees receiving Fresenius Kabi Propoven 2% Emulsion will track all medication errors associated with the use of and all serious adverse events that are considered to be potentially attributable to Fresenius Kabi Propoven 2% Emulsion use and must report these to FDA using one of the following methods:
- Complete and submit a MedWatch form online (www.fda.gov/medwatch/report.htm)
- Complete and submit FDA Form 3500 (health professional) by fax (1-800-FDA-0178) (this form can be found via link above).
Call 1-800-FDA-1088 for questions. Submitted reports should state, “use of Fresenius Kabi Propoven 2% Emulsion was under an EUA” at the beginning of the question “Describe Event” for further analysis.
To report adverse events to Fresenius Kabi, contact the Vigilance department at Fresenius Kabi USA by calling 1-800‐551‐7176, option 5 or by email adverse.events.USA@Fresenius Kabi-kabi.com.
To report a product complaint to Fresenius Kabi, contact the Medical Affairs Department at Fresenius Kabi USA by calling 1-800-551-7176, option 1 or by email productcomplaint.USA@Fresenius Kabi-kabi.com.
To ask questions about the safety, efficacy or use of a Fresenius Kabi product, contact the Medical Affairs Department at Fresenius Kabi USA by calling 1-800-551-7176, option 3, or by email medinfo.USA@fresenius-kabi.com.
To ask about Fresenius Kabi pharmaceutical product availability and ordering, contact Fresenius Kabi USA Customer Service by calling 1-888-386-1300 or by email customerservice.USA@fresenius-kabi.com.
Fresenius Kabi USA Vigilance, Medical Affairs and Customer Service are available Monday through Friday, between the hours of 8 a.m. and 5 p.m. (CST).
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany.
Contacts
Matt Kuhn, (847) 550-5751
matt.kuhn@Fresenius Kabi-kabi.com