US clinical trial site concerns over COVID-19 impact on enrollment jumps by 124% over past week
US clinical trial site concerns over COVID-19 impact on enrollment jumps by 124% over past week
European site enrollment concerns grow to 85%
NORTHBROOK, Ill.--(BUSINESS WIRE)--Over the course of just one week, US clinical research study sites’ concern about COVID-19-related interruptions to recruitment and retention has increased by 124%, jumping from 25% to 56% according to an ongoing quantitative survey conducted by Continuum Clinical. Clinical trial sites in Europe who responded to the survey continue to indicate a higher level of concern overall, with nearly 85% of sites indicating the pandemic will negatively impact clinical trial enrollment.
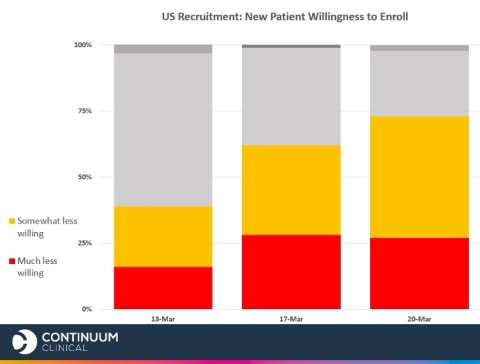
On March 20, Continuum Clinical, a global clinical trial enrollment company specializing in fact-based strategies, gathered follow up results from their survey fielded previously on March 13 and 17. New results indicate continuing worrisome trends among both US and European clinical trial sites across the board: 73% of responding US sites and 84% of responding European sites said the novel coronavirus will negatively impact patient willingness to enroll in clinical trials.
“Looking at these trends change over the week shows just how quickly and dramatically outlooks have shifted,” said Continuum Clinical president Neil Weisman. “Clinical research sites, in this case, are following the trends we are seeing everywhere – negative outlooks, increased fears, growing uncertainty. It’s important for our sponsor and CRO partners to be aware of these trends so they can respond with clear guidance in this unprecedented situation.”
March 13 survey results showed one third (29%) of US sites anticipating COVID-19 will have a “big or extremely big impact” on patient recruitment and retention – that number jumped to 44% as of March 20.
Retention concerns continue to grow as well, with 56% of US sites and 81% of European sites indicating already-enrolled patients are “much less or somewhat less likely” to continue participating in trials.
“Research sites are feeling the biggest immediate impact of COVID-19,” said Continuum Clinical vice president of Data and Analytics Paul Ivsin. “A jump in site concern this significant in just a week should be getting the attention of every trial sponsor in the industry.”
In an effort to continue monitoring the impact COVID-19 has on clinical research, including identifying emerging trends, Continuum Clinical is committed to monitoring developments as they pertain to patient recruitment and retention, and posting updates that can help the industry navigate these difficult times.
About Continuum Clinical
Continuum Clinical is a global clinical trial enrollment company, providing fact-based patient recruitment solutions that deliver results. With over twenty-five years of experience, Continuum Clinical provides sponsors and CROs with patient recruitment and retention planning, study and site support, patient recruitment campaigns, patient advocacy and diversity & inclusion services, retention solutions, and reporting and analytics. We specialize in identifying and solving challenges that can impact successful clinical trial enrollment, from protocol development through study completion. Headquartered in the US, Continuum Clinical has more than 100 employees in the US and Europe and an expanded network of resources worldwide.
Contacts
Courtney Cymerman
ccymerman@continuumclinical.com
Media Contact
847-418-8040