Paragon 28 Launches Comprehensive Supramalleolar Osteotomy System
Paragon 28 Launches Comprehensive Supramalleolar Osteotomy System
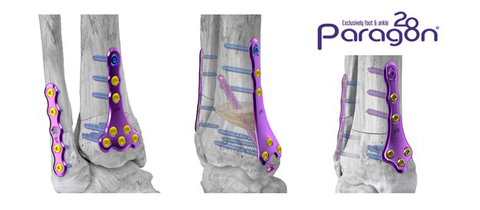
ENGLEWOOD, Colo.--(BUSINESS WIRE)--Paragon 28, Inc. (NYSE: FNA) is pleased to announce the launch of its Gorilla® Supramalleolar Osteotomy (SMO) Plating and PRESERVE™ SMO Allograft System which were developed to provide surgeons versatility in plate selection and surgical approach for supramalleolar osteotomies. The system includes patent pending drilling and cutting guides to facilitate repeatable and controlled anterior dome, medial opening, and closing wedge osteotomies. The system also includes a patent pending allograft cutting jig designed to shape the PRESERVE™ SMO Allograft Wedge to match the intended correction while reducing time and technicality of the procedure. Six plates are included in the system for anterior or medial approaches and include multiple spanning distances to accommodate varying correction.
Mark Myerson, MD, Gorilla® SMO design surgeon, said, "Paragon28 has introduced an outstanding and precise supramalleolar osteotomy plating system. It is extremely accurate, enabling the surgeon to obtain optimal correction with cutting and drill guides and a truly unique and ingenious way to cut a PRESERVE™ Allograft Wedge for an opening wedge osteotomy."
Paragon 28’s CEO, Albert DaCosta, commented, “Hindfoot deformity correction and arthroplasty are two of the fastest growing segments in the foot and ankle market and the Gorilla® SMO Plating and Allograft System offers surgeons a streamlined and reproducible means to achieve deformity correction while preserving the ankle joint. We are very excited to have a comprehensive system which will allow surgeons to address tibial deformities and stage for total ankle arthroplasty supporting our growing APEX™ Total Ankle Arthroplasty System.”
The addition of the Gorilla® SMO Plating and Allograft System bolsters Paragon 28’s Precision Ankle Solutions product offering, which includes the Gorilla® Ankle Fracture Plating System, APEX 3D™ Total Ankle Replacement, Silverback™ Ankle Fusion Plating System, Phantom® TTC Nail System, and Phantom® ActivCore™ Nail System. With this comprehensive portfolio, Paragon 28® provides its customers with innovative ankle solutions for trauma, arthritis, and limb salvage.
Paragon 28® is grateful for the significant contributions that Woo Chun Lee, MD, Mark Myerson, MD, Cassandra Tomczak, DPM, and Federico Usuelli, MD made in the design of this system.
About Paragon 28, Inc.
Based in Englewood, CO., Paragon 28, is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments including fracture fixation, forefoot, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, Charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes, reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
Contacts
Investor Contact:
Matt Brinckman
Senior Vice President, Strategy and Investor Relations
(720) 912-1332
mbrinckman@paragon28.com