Omeros Corporation Reports Interim Data with Alternative Pathway Inhibitor OMS906 as Monotherapy in Patients with Paroxysmal Nocturnal Hemoglobinuria
Omeros Corporation Reports Interim Data with Alternative Pathway Inhibitor OMS906 as Monotherapy in Patients with Paroxysmal Nocturnal Hemoglobinuria
At lowest planned subcutaneous dose, Omeros’ MASP-3 inhibitor OMS906 showed clinically and statistically significant improvements in hemoglobin and LDH, which were seen early and maintained throughout the observation period
- Following 2 doses of OMS906, all patients achieved an increase in hemoglobin of ³ 4.0 g/dL, with a mean hemoglobin change from baseline of 4.75 g/dL (p < 0.001)
- Following 3 doses of OMS906, mean hemoglobin increase from baseline was 6.27 g/dL (p = 0.005)
- All OMS906-treated patients remained transfusion-free throughout the observation period
- OMS906 was well tolerated with no safety signals of concern
SEATTLE--(BUSINESS WIRE)--Omeros Corporation (Nasdaq: OMER) today announced positive results from a pre-specified interim analysis of its ongoing Phase 1b clinical trial of OMS906, the company’s lead MASP-3 inhibitor, in complement-inhibitor-naïve adults with paroxysmal nocturnal hemoglobinuria (PNH), a rare and life-threatening hemolytic blood disorder. Statistically significant and clinically meaningful improvements were observed in all measured markers of hemolysis, including hemoglobin (Hgb) and lactate dehydrogenase (LDH). To date, all patients have received only the lowest subcutaneous dose of OMS906 in this multidose trial.
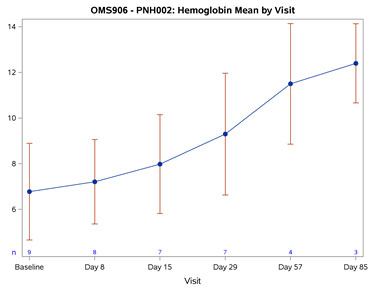
The baseline mean Hgb was 6.78 g/dL. By Day 57 (after 2 doses), all OMS906-treated patients achieved an increase in Hgb of 4.0 g/dL or more, with a mean change from baseline of 4.75 g/dL (p < 0.001). By Day 85 (following 3 doses), the mean Hgb was 12.4 g/dL with a mean change from baseline of 6.27 g/dL (p = 0.005). Improvement was rapid, with significant mean Hgb improvement of 0.88 g/dL (p = 0.003) seen at the first timepoint (Day 8), persistently increasing and remaining statistically significant through the last observed timepoint (Day 85). No patients required or received blood transfusions following OMS906 treatment initiation. Figure 1 demonstrates the Hgb improvement.
The mean baseline LDH was 1931, nearly 8 times the upper limit of normal. Statistically significant improvements in LDH were observed at Day 8, the first measured timepoint, with subsequent further and statistically significant reductions seen throughout the observation period.
OMS906 has been safe and well tolerated in this trial, consistent with Phase 1 safety observations in healthy subjects.
This single-arm, open-label clinical trial is evaluating the effect of once-monthly subcutaneous administration of OMS906 in patients with PNH. A total of 9 patients have been enrolled to date and all have been complement-inhibitor-treatment naïve. Co-existing conditions in these 9 patients include aplastic anemia, iron deficiency, myelodysplastic syndrome, and chronic renal failure. To date, 7 patients have received 2 or more doses of OMS906, 4 have received 3 or more doses, and 3 have received 4 doses.
Omeros has established a broad intellectual property estate directed to the inhibition of MASP-3. OMS906 is Omeros’ lead investigational humanized monoclonal antibody targeting MASP-3, the key and most upstream activator of the alternative pathway of complement. MASP-3 circulates in low concentrations and has slow turnover, allowing consistent and long-duration target coverage and pathway inhibition. Unlike other alternative and terminal complement pathway inhibitors on the market or in development, MASP-3 inhibition leaves the infection-fighting function of the classical pathway intact and, given that MASP-3 is known not to be an acute phase reactant, has less risk of breakthrough occurrence of the underlying disease. Inhibition of MASP-3, which is located upstream of complement component 5 (C5), C3, Factor B and Factor D, is expected to block both intra- and extravascular hemolysis in PNH.
Based on pharmacokinetic data in both healthy subjects and patients with PNH and the efficacy data observed in PNH patients, Omeros is targeting a dosing frequency of once quarterly either intravenously or subcutaneously. This dosing frequency and the extended OMS906 half-life should provide excellent patient convenience and compliance as well as additional protection from pharmacokinetic or pharmacodynamic breakthrough, further enhancing efficacy.
“These trial data are quite impressive,” said Eleni Gavriilaki, M.D., Ph.D., assistant professor of hematology at Aristotle University, Thessaloniki, Greece. “The effect of OMS906 in PNH, a disease that requires a high level of alternative pathway suppression to see clinical benefit, bodes well for the drug’s role in treating any disorder associated with dysregulation of the pathway. Achievement of once-quarterly dosing would be well received by physicians and their patients, representing a paradigm shift in the treatment of PNH and other alternative pathway disorders.”
Omeros’ second OMS906 clinical trial in PNH patients, treating those who have demonstrated an inadequate response to ravulizumab, a C5 inhibitor, just recently entered the OMS906 dosing phase and is ongoing. The OMS906 clinical trial in C3 glomerulopathy is also underway. Preliminary data from both of these trials are expected in the third quarter of this year. Omeros is also preparing to initiate a clinical trial assessing once-quarterly systemic delivery of OMS906, avoiding the need for intravitreal delivery, in patients with geographic atrophy, an advanced form of dry age-related macular degeneration (AMD).
“We are excited by the data resulting from this trial of treatment-naïve patients,” said Gregory A. Demopulos, M.D., Omeros’ chairman and chief executive officer. “The degree of hemoglobin improvement in such a severely anemic population is remarkable. The results observed to date demonstrate that OMS906 provides clinically effective inhibition of the alternative pathway, opening for the drug the wide range of alternative pathway-related diseases, a good number of which already have been clinically validated by agents on the market or in development. We believe that the strong proof-of-concept data generated, the scope of already-validated alternative pathway-related diseases, and the expected advantages of OMS906 over other alternative pathway inhibitors make clear the drug’s substantial commercial potential.”
Omeros will present detailed clinical trial data, including data in this preliminary analysis, at an upcoming international conference. Omeros is also amending both of its ongoing Phase 1b clinical trials in PNH to Phase 2, expanding enrollment, assessing planned higher doses, and making preparations to meet with regulatory authorities to discuss the clinical development plan to approval.
About Omeros Corporation
Omeros is an innovative biopharmaceutical company committed to discovering, developing and commercializing small-molecule and protein therapeutics for large-market and orphan indications targeting immunologic disorders including complement-mediated diseases, cancers, and addictive and compulsive disorders. Omeros’ lead MASP-2 inhibitor narsoplimab targets the lectin pathway of complement and is the subject of a biologics license application pending before FDA for the treatment of hematopoietic stem cell transplant-associated thrombotic microangiopathy (TA-TMA). Narsoplimab is also in multiple late-stage clinical development programs focused on other complement-mediated disorders, including IgA nephropathy, COVID-19, and atypical hemolytic uremic syndrome. Omeros’ long-acting MASP-2 inhibitor OMS1029 is currently in a Phase 1 clinical trial. OMS906, Omeros’ inhibitor of MASP-3, the key activator of the alternative pathway of complement, is advancing in clinical programs for paroxysmal nocturnal hemoglobinuria (PNH), complement 3 (C3) glomerulopathy and other related indications. For more information about Omeros and its programs, visit www.omeros.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, which are subject to the “safe harbor” created by those sections for such statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “likely,” “look forward to,” “may,” “objective,” “plan,” “potential,” “predict,” “project,” “should,” “slate,” “target,” “will,” “would” and similar expressions and variations thereof. Forward-looking statements, including statements regarding anticipated safety and therapeutic benefits of Omeros’ drug candidates are based on management’s beliefs and assumptions and on information available to management only as of the date of this press release. Omeros’ actual results could differ materially from those anticipated in these forward-looking statements for many reasons, including, without limitation, unanticipated or unexpected difficulties in the conduct of planned research, unproven preclinical and clinical development activities, changes in our financial condition and results of operations, challenges associated with manufacture or supply of our investigational drug candidates, intellectual property claims, competitive developments, litigation, and the risks, uncertainties and other factors described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 13, 2023. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and the company assumes no obligation to update these forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
Contacts
Jennifer Cook Williams
Cook Williams Communications, Inc.
Investor and Media Relations
IR@omeros.com