CMIC Supports Clinical Trials for Digital Therapeutics Using SUSMED’s Trial Management System
CMIC Supports Clinical Trials for Digital Therapeutics Using SUSMED’s Trial Management System
TOKYO--(BUSINESS WIRE)--CMIC Co., Ltd. (henceforth “CMIC”)(TOKYO:2309) has launched services using SUSMED, Inc.’s (henceforth “SUSMED”) trial management system covering subject registration, software assignment and distribution for digital therapeutics (DTx).
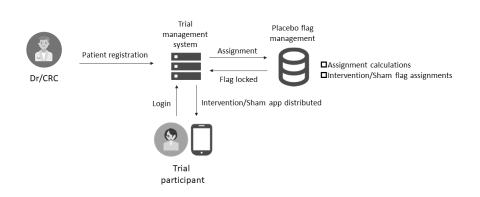
The system is able to overcome the common challenges of DTx clinical trials, ensuring blinded assignment and preventing unauthorized access. By employing this system, CMIC will offer more efficient operations for DTx trials, such as for the assignment and delivery of software applications (apps) to trial participants, cutting development costs.
In a DTx clinical trial, the software app must be installed on a device, such as a smartphone. Additionally, a placebo app (sham) is often used as a control in comparison to the actual treatment app (intervention), so it is essential that each app is correctly distributed to each patient, as assigned. For this reason, many processes and tasks differ from general drug development, such as loaning pre-installed, study-specific devices to trial subjects. This results in a heavy burden on companies developing DTx. Furthermore, because everything from assignment to patient distribution is done digitally, it is vital to take security measures in order to prevent any unauthorized access.
When using SUSMED’s system, each patient will be registered in a secure environment, and an app (intervention or sham) will be assigned to each patient with a specific ID. The patient can start his or her assigned treatment by installing the app using the issued ID. As this ID-assignment function allows patients to install their assigned app to their personal device, device rental is unnecessary, cutting development costs significantly. In addition, higher quality data and treatment compliance is expected because patients are already familiar with their own device.
Making the most out of the system’s features, CMIC will effectively complete each DTx clinical trial by formulating the best distribution strategies for each therapeutic intervention.
“We have developed this service as an extension of the strategic partnership with SUSMED that we announced on October 21st this year. By introducing SUSMED’s trial management system, we hope to overcome some of the security and cost challenges of DTx development. We will continue to promote open innovation and the growth of the DTx market, in order to answer the needs of clients, healthcare providers and patients, as soon as possible.” said Toru Fujieda, President of CMIC Co., Ltd.
"Since its founding, SUSMED has developed new technology in order to achieve sustainable medicine through ICT. There are several challenges in DTx development that differ from drug development. By providing this trial management system, we hope to contribute to faster and more efficient development of DTx. We will continue to contribute to the growth of DTx by combining SUSMED’s technology and CMIC’s comprehensive clinical trial services.” said Taro Ueno, CEO of SUSMED, Inc.
To learn more about this service, please contact us at dtx_prgroup@cmic.co.jp
●About CMIC group
CMIC Group was founded in 1992 as the first Contract Research Organization (CRO) in Japan. Today CMIC Group is the largest clinical CRO in Japan with global footprint, providing comprehensive services in drug development, clinical site management, clinical to commercial GMP manufacturing, regulatory consulting and contract sales & marketing solutions. We can help pharmaceutical, biotech and medical device companies to enter Japan market, to conduct clinical trials in Asia, or to bridge drug development and manufacturing needs in the US, Europe, Japan and broader Asia. CMIC Group has over 7,000 employees and 25 sites globally. For more information about CMIC Group and services, please visit our website.
https://en.cmicgroup.com/
●About SUSMED, Inc.
SUSMED Inc., is a research and development firm that is working to advance digital therapies. In addition to developing an app for treating insomnia, SUSMED provides a universal platform for developing medical apps, along with clinical trial support systems and automated AI analysis systems. SUSMED is advancing digital therapy grounded in technology, with patents in medical apps and blockchain applications in medical treatment.
https://www.susmed.co.jp/en
Contacts
CMIC HOLDINGS Co., LTD.
PR group
Yuko Ishikawa
E-mail: pr@cmic.co.jp
SUSMED, Inc.
E-mail: support@susmed.co.jp