ENFit™ Components from Qosina Help Reduce Critical Enteral Misconnections
ENFit™ Components from Qosina Help Reduce Critical Enteral Misconnections
RONKONKOMA, N.Y.--(BUSINESS WIRE)--Qosina is the trusted supplier of enteral components for medical devices, offering over 250 off-the-shelf products including a wide selection of legacy feeding tube connectors and fittings.
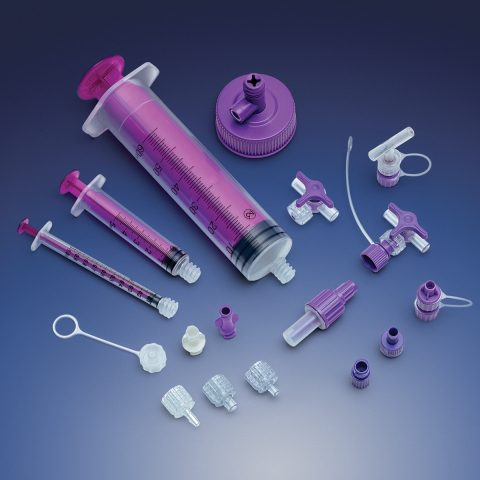
Also included in Qosina’s enteral application product line is its robust collection of ENFit™ connectors, caps and plugs, stopcocks, spikes, and syringes. ENFit™ components comply with the ISO 80369-3 standard, which was developed to improve patient safety and reduce the risk of small-bore misconnections used in liquid healthcare applications.
The ENFit™ connector was designed using a non-traditional orientation and larger dimensions that do not allow connectivity with the common conical luer (formerly ISO 594, now ISO 80369-7). The ENFit™ connector has been tested using a strict validation process that includes human factors research, usability analysis, performance testing, misconnections assessment and computer-aided design techniques.
To learn more about Qosina’s enteral and ENFit™ products, visit https://www.qosina.com/enteral. Qosina adheres to the strictest of quality standards and is committed to providing high quality products.
Founded in 1980, Qosina is a leading global supplier of OEM single-use components to the medical and pharmaceutical industries. Qosina’s philosophy is to address its customers’ need to reduce time to market by providing thousands of stock components. The company’s vast catalog features more than 5,000 products shown in full-scale illustrations on a one-centimeter grid. Qosina offers free samples of most items, low minimum order requirements, just-in-time delivery, modification of existing molds, and new product design and development. Qosina is ISO 13485, ISO 9001, ISO 22301 and ISO 14001 certified, and operates in a 95,000 square-foot facility with an ISO Class 8 Clean Room. To learn about Qosina’s full component offering, which includes the newest products, visit www.qosina.com or call +1 (631) 242-3000. Visit Qosmedix, Qosina’s cosmetics division, at www.qosmedix.com. Qosmedix is a certified global supplier of beauty tools and accessories to the cosmetic, skincare, spa and salon industries.
Contacts
Qosina Corp.
Rachelle Morrow
+1 (631) 242-3000
rmorrow@qosina.com