Integrated Medical Professionals, a Multi-Specialty Physician Group and Affiliate of Solaris Health, Adopts Ambu’s Single-Use Cystoscopy
Integrated Medical Professionals, a Multi-Specialty Physician Group and Affiliate of Solaris Health, Adopts Ambu’s Single-Use Cystoscopy
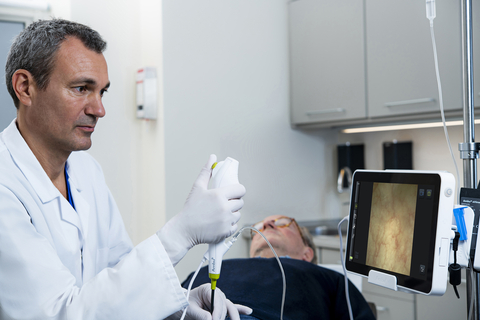
COLUMBIA, Md.--(BUSINESS WIRE)--Ambu Inc., the world leader in single-use endoscopy, announced today that Integrated Medical Professionals PLLC, a multi-specialty physician group operating in the New York metropolitan area, is adopting Ambu’s aScope™ 4 Cysto and aView™2 Advance HD Monitor.
IMP was formed in 2006 by 31 physicians from 13 different practices and has grown to 100 physicians seeing patients at nearly 50 sites in the New York metro area. Dr. Kathleen L. Latino, IMP’s medical director and a urologist, sees the single-use adoption as a step forward for both physicians and patients, citing the potential of single-use cystoscopy to improve efficiency and workflow in their clinics while providing a better patient experience.
“We are excited about our partnership with Ambu, the most innovative single-use endoscopy company,” Dr. Latino said. “IMP is constantly looking for new innovations and technologies that allow us to become more efficient and provide better patient care. We have been highly impressed with the performance of Ambu’s disruptive and high-quality single-use cystoscope platform. It allows us to eliminate costly repairs, cumbersome and time-consuming reprocessing, and enables improved workflow efficiency.”
Flexible cystoscopes were recently classified as high-risk flexible endoscopes by the Association for the Advancement of Medical Instrumentation® (AAMI), with the recommendation to sterilize reusable devices between patient uses, when possible.
The U.S. Food and Drug Administration announced in April 2021 that it was investigating “numerous” medical device reports (MDRs) describing patient infections and other contamination issues possibly associated with reprocessed urological endoscopes, including cystoscopes.
With reprocessing guidelines becoming more comprehensive and stringent, single-use cystoscopy is an attractive and cost-effective alternative for both office and hospital-based clinics.
“We are thrilled that IMP has embraced Ambu’s sterile single-use aScope 4 Cysto and has already used it successfully on many patients,” Ambu President Steven Block said. “We are continuing to see rapid adoption of the aScope 4 Cysto across health care facilities in the U.S. and are excited to see the positive impact for hospitals, clinics, physicians, and staff.”
The aScope™ 4 Cysto and aView 2 Advance are on contract with all major U.S. Group Purchasing Organizations (GPO).
About Ambu
Ambu has been bringing the solutions of the future to life since 1937. Today, millions of patients and healthcare professionals worldwide depend on the efficiency, safety and performance of our single-use endoscopy, anaesthesia, and patient monitoring solutions. We continuously look to the future with a commitment to deliver innovative quality products that have a positive impact on patient care and the work of healthcare professionals. Headquartered near Copenhagen in Denmark, Ambu employs approximately 4,500 people in Europe, North America and the Asia Pacific. For more information, please visit ambu.com or ambuUSA.com.
Contacts
U.S. media
Klaas-Pieter Jimmink, Marketing Communications Director, tel. 443-766-1363, email: kjim@ambu.com
Ambu Inc., 6230 Old Dobbin Lane, Suite 250, Columbia, Maryland, 21045, United States, www.ambuusa.com
European and APAC media
Mikkel Trier Wagner, Director Corporate Communications, tel. +45 4191 0830, email: mtw@ambu.com
Ambu A/S, Baltorpbakken 13, 2750 Ballerup, Denmark, Tel. +45 7225 2000, CVR no.: 63 64 49 19, www.ambu.com