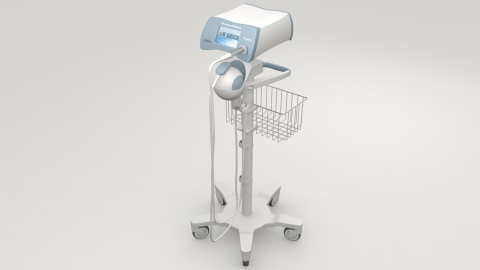
CARLSBAD, Calif.--(BUSINESS WIRE)--RF Surgical Systems Inc. (RFS), the market leader in adjunct detection technology for the prevention of retained surgical sponges, announces the availability of the RF Assure® Delivery System. This is the first device on the market for locating and preventing sponges and gauze exclusively designed for the labor and delivery environment. The RF Assure Delivery System, featuring the Verisphere, began shipping this month to hospital and medical center labor and delivery departments.
The patented RF Assure Delivery System uses low energy radio frequency (RF) tags in cotton surgical sponges, gauze and towels commonly used in labor and delivery and features the Verisphere sensor to quickly and unobtrusively scan a patient to detect sponges in the birthing canal as well as around the room.
Most hospitals in the U.S., particularly labor and delivery rooms, rely on traditional manual counting protocols to detect and prevent the occurrences of retained surgical sponges. However, a growing body of clinical evidence1 indicates that manual sponge counts alone are not sufficient to protect patients from these “never events.” An estimated 22 percent of retained surgical sponges — more than 1,000 annually — are accidentally left inside patients during standard vaginal deliveries2.
Importantly, in up to 97 percent of cases3, labor and delivery department sponge counting policies are cited as the top risk factor in retained surgical sponges following vaginal deliveries. According to The Joint Commission, the nation’s oldest and largest standard-setting and accreditation body in healthcare, retained vaginal sponges are reviewable as a “sentinel event,” and can be reported as a breach in quality and patient safety measures4.
“The prevalence of retained surgical sponges in vaginal deliveries is unacceptable, particularly in light of the availability of cost-effective adjunct detection technology that is clinically proven to help ensure the safety of birth mothers,” said Dr. Hector Chapa, FACOG, clinical faculty at Methodist Medical Center Dallas, and Medical Director of Women’s Specialty Center Dallas. “Obstetric practitioners need to look to these devices as the new standard of care to increase the confidence of labor and delivery staff, improve quality of patient care and reduce overall liability.”
Unlike the standard RF Assure Detection System that uses a mat and wand to track surgical sponges in patients who are lying down, the RF Assure Delivery System is optimized for women in child birth position. After birth, clinicians simply and unobtrusively scan the mother’s pelvic region and then the Labor and Delivery room. A simple 15-second scan gives staff the confidence of an accurate sponge count.
“Most doctors and parents agree the birth of a child is a family’s most important event. Our goal is to make sure retained surgical sponges don’t harm that experience or cause avoidable health problems,” said RF Surgical CEO John Buhler.
About RF Surgical Systems, Inc.
RF Surgical Systems, Inc. is the market leader in the detection and prevention of retained surgical items. The company’s flagship product, the RF Assure® Detection System, is the preferred solution in more than 4,500 OR, trauma, and L&D suites nationwide, including most top ranked U.S. hospitals and trauma centers.
RF Surgical Systems is based in Carlsbad, California. U.S. patents protect the advanced technologies used in the RF Assure Detection System™. Regulatory clearance to market the system was granted by the U.S. Food and Drug Administration in November 2006. Additional information about RF Surgical Systems, Inc. can be found at www.rfsurg.com.
1 Journal of the American College of Surgery, March 2014
2 Gawande, 2003, NEJM
3 Spotlight on Patient safety, Data, Trends, and Learning from the Minnesota Adverse Health Events Reporting System April 2009 www.health.state.mn.us/patientsafety
4 http://www.jointcommission.org/about/JointCommissionFaqs.aspx?faq#69