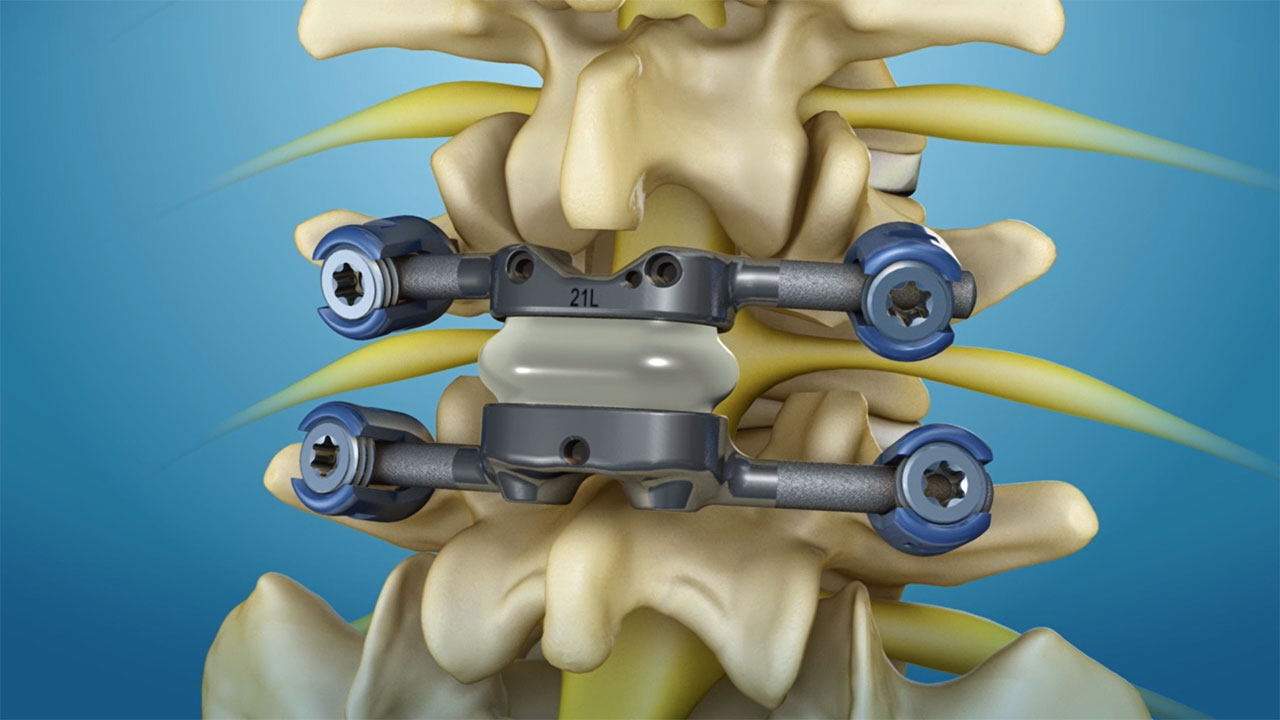
NORWALK, Conn.--(BUSINESS WIRE)--Premia Spine, a medical technology company aiming to change the way debilitating chronic leg and back pain is treated, today announced that its TOPS facet arthroplasty system won Breakthrough Device Designation from the U.S. Food & Drug Administration.
TOPS is the first and only facet joint replacement system for the lumbar spine. Developed to offer a new treatment option for patients suffering from spinal stenosis or spondylolisthesis, the system is designed to provide mobility, stability, and durability after decompression. TOPS has been proven in early clinical studies to deliver immediate and sustained pain relief and improvement in patient quality of life, and is currently the subject of a pivotal clinical trial under an investigational device exemption from the FDA.
“The TOPS System is designed to relieve debilitating lower back and leg pain while enabling long-term mobility, stability, and durability for patients,” said Premia Spine CEO Ron Sacher. “We are gratified that the FDA recognizes TOPS’s potential to advance the standard of care for patients with spinal stenosis and spondylolisthesis.”
Breakthrough designation expedites the development and FDA review of new devices with the potential to more effectively treat or diagnose life-threatening or irreversibly debilitating conditions. FDA recognition of the TOPS System as a Breakthrough Device means Premia Spine will receive feedback throughout the regulatory process, as well as prioritized review by the agency.
“TOPS has the potential to fill a void in our treatment armamentarium for spinal stenosis and spondylolisthesis. If the results of the pivotal study mirror those found in early clinical trials, TOPS could be a game changer for surgeons and their patients,” said Dr. Dom Coric, chief of neurosurgery at Carolinas Medical Center in Charlotte and lead investigator in the U.S. pivotal trial. “There's no other posterior arthroplasty device for the lumbar spine. Like most surgeons, I welcome the opportunity to advance spinal therapy with pioneering solutions like TOPS.”
About Premia Spine
Premia Spine, a medical technology company, aims to improve the lives of chronic leg and back pain patients with its TOPS™ System facet arthroplasty system. TOPS is designed to provide lasting mobility, stability and durability to patients with lumbar spinal stenosis, degenerative spondylolisthesis and related spinal conditions. Premia Spine is headquartered in Netanya, Israel, with U.S. operations based in Norwalk, CT. For more information, visit premiaspine.com.
Disclaimer: The TOPS System is limited by Federal (or United States) law to investigational use only.