NANJING, China--(BUSINESS WIRE)--IASO Biotherapeutics (IASO BIO), a clinical stage biotechnology company advancing the development of innovative therapies for cancer, and Innovent Biologics, Inc. (Innovent) (HKEX:01801), a world-class biopharmaceutical company that develops and commercializes high quality medicines, announced today that IASO BIO has received National Medical Products Administration (NMPA) approval for an Investigational New Drug Application (IND) for CT103A—an innovative therapy for the treatment of relapsed refractory multiple myeloma (rr/mm) patients. IASO Bio and Innovent will start a Phase Ib/II study to confirm the R2PD and move to phase II shortly after, with approval anticipated in 2021.
Multiple Myeloma is a deadly blood cancer that often infiltrates the bone marrow causing anemia, kidney failure, immune problems and bone fractures. With a global annual incidence rate of 2/100,000 persons, it is one of the most commonly diagnosed blood cancers, second only to non-Hodgkin lymphoma.
Hu Guang, Ph.D., Director of R&D at IASO BIO, commented that: "This is an important milestone that allows us to continue with CT103A’s clinical development for the treatment of rr/mm. We’re a young company, and this is a big step forward in achieving our objectives to develop and bring to market the most advanced therapies for treating patients more effectively.”
News of the IND approval coincided with the latest presentation of CT103A clinical results at the 17th Annual International Myeloma Workshop, Boston, September 12-15 (Abstract #440).
This annual event is devoted to fostering scientific and clinical exchange on the latest breakthroughs in multiple myeloma and related plasma cell disorders.
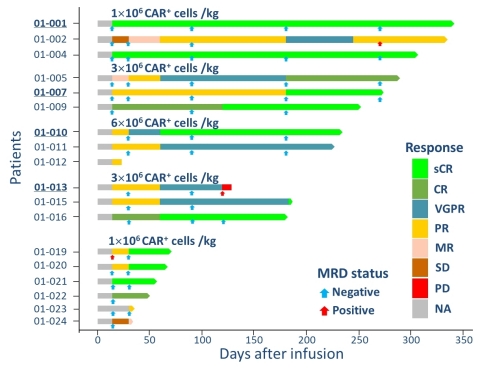
In an IIT study conducted by Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, researchers presented compelling results for efficacy and persistence of a therapy that may provide patients, having relapsed from a prior CAR-T, an option for CAR-T retreatment.
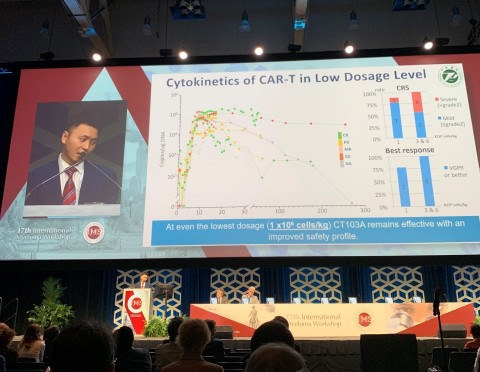
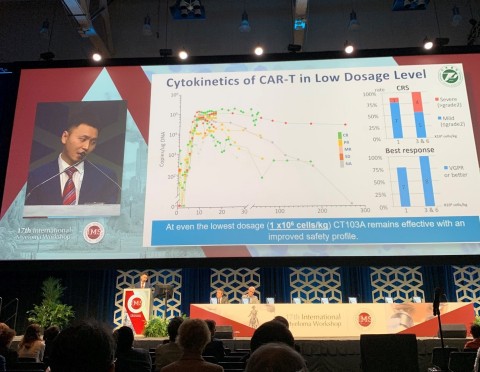
In general, the treatment was well tolerated, with dosage levels ranging from 1X106, 3X106 and 6X106 CAR-T/kg.
CRS occurred in 17/18 patients (Grade 1&2- 66.6% (12), Grade 3- 22.2% (4), Grade 4- 5.6% (1)), but was generally manageable with no neurotoxicity.
At even the lowest dosage level (1 x106 cells/kg), CT103A remains effective with an improved safety profile. For response, 7/8 evaluable patients achieved VGPR or better. With respect to safety, only 1/8 evaluable patients experienced CRS at > grade 2.
About CT103A:
CT103A is an innovative therapy co-developed by IASO BIO and Innovent Biologics, Inc. Previous studies indicated patients with relapsed/refractory multiple myeloma (RRMM) who received high-dose BCMA-targeting CAR-T cells may achieve better remission but have worse adverse events. Moreover, once the disease progresses, the re-infusion of CAR-T cells is not effective. To solve this dilemma, CT103A has been developed, a lentiviral vector containing a CAR structure with a fully human scFv, CD8a hinger and transmembrane, 4-1BB co-stimulatory and CD3z activation domains. Based on strict selection and screening, utilizing a proprietary in-house optimization platform, the construct of the CT103A CAR-T is potent and persistent.
About Nanjing IASO Biotherapeutics:
Founded in March 2017, IASO BIO is a clinical stage biotechnology company advancing the development of innovative cell therapies for cancer. IASO BIO is dedicated to curing cancer using engineered autologous/allogenic T cell therapies designed to enhance the immune system's ability to recognize and eradicate cancer cells.
Currently, IASO BIO is developing over 10 high-potential, high-end biopharmaceutical products, utilizing a fully-human scFv sequence targeting hematological, solid and virus associated tumors. Additional development efforts include unique TCR-like CAR-T cell therapy products for indications such as gastric cancer, nasopharyngeal carcinoma and viral infection related solid tumors.
IASO BIO has developed broad capability including; a proprietary phage library(>1x1011)supporting the development of CAR-T products, antibody drugs and intracellular targets, a screening system using high-throughput CAR-T analysis techniques and large scale data mining to obtain Best-in-class CAR-T drug candidates, in-house plasmid, lentivirus and CAR-T production technology platforms meeting the requirements for IND submissions and clinical research. For more information, please visit: www.iasobio.com
About Innovent
Inspired by the spirit of "Start with Integrity, Succeed through Action,” Innovent’s mission is to develop and commercialize high quality biopharmaceutical products that are affordable to ordinary people. Established in 2011, Innovent is committed to developing, manufacturing and commercializing high quality innovative medicines for the treatment of oncology, metabolic disorders, and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since it was founded, Innovent has developed a fully-integrated platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities. Leveraging the platform, the company has built a robust pipeline of 21 innovative assets in the fields of oncology, metabolic diseases and other major therapeutic areas. Sixteen have entered into clinical development, four have entered Phase 3 clinical trials, three monoclonal antibodies have their New Drug Application (NDA) under review and three of them have been granted with priority review status, and one, Tyvyt® (sintilimab injection), is now approved for relapsed or refractory classical Hodgkin’s lymphoma (r/r cHL).
Innovent has built an international team of advanced talents in high-end biological drug development and commercialization, including many overseas experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Adimab, Incyte, Hanmi and other international pharmaceutical companies. Innovent strives to work with all relevant parties to help advance China’s biopharmaceutical industry, improve drug availability to ordinary people and enhance the quality of the patients’ lives. For more information, please visit:www.innoventbio.com.