LYON, France & NEW YORK--(BUSINESS WIRE)--The Medicrea Group (Euronext Growth Paris - FR0004178572 - ALMED; OTCQX Best Market – MRNTF), pioneering the digital transformation of spinal surgery through artificial Intelligence, predictive modeling and patient specific implants with its UNiD ASI™ (Adaptive Spine Intelligence) proprietary software platform, services and technologies, publishes sales for the first half of 2019.
(€ millions) |
H1 2018 |
H1 2019 |
Variation |
Variation
|
USA Rest of the world |
7.6 7.0 |
8.7 7.4 |
+15% +6% |
+7% +6% |
Total Sales – Comparable basis Discontinued activities Total Sales |
14.6 2.3 16.9 |
16.1 - 16.1 |
+10% - (5)% |
+6% - (9)% |
Sales for the first half of 2019 amounted to 16.1 million euros, up 10% (+6% at constant exchange rates) compared to 2018 on a pro-forma basis. In the United States, the Group's most significant market, sales growth continued with an increase of 15% compared to 6% for the rest of the world (respectively + 7% and + 6% at constant exchange rates). As previously mentioned, Medicrea discontinued non-strategic activities on July 1st, 2018, which represented 2.3 million euros of sales in the first half of 2018, showing a total decrease in sales of 5% over the period. These activities will no longer impact the period-over-period comparisons effective the second half of 2019.
The first half of 2019 demonstrated record activity for UNiD™ patient-specific rods. The milestone of 4,000 surgeries performed has been achieved, with 423 surgeries in the 2nd quarter of 2019. In the month of June alone, 171 personalized UNiD™ surgeries were performed and a new monthly record should be established in July.
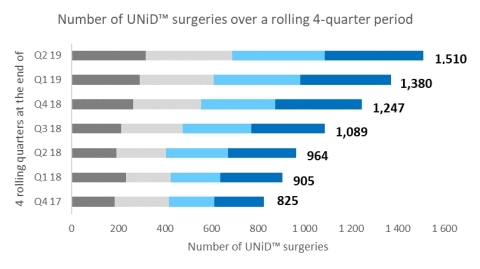
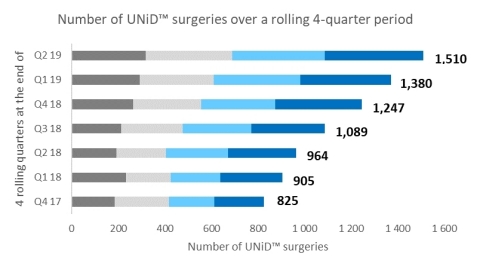
The adoption of UNiD ASI™ technology for patient-specific spinal surgery is accelerating rapidly quarter-over-quarter. The following graph shows the evolution of total UNiD™ surgeries over a rolling 4-quarter period. Over the last 18 months, the increase exceeds 80% (825 surgeries performed over the last 4 quarters at the end of Q4 2017 versus 1,510 at the end of Q2 2019).
Outlook
The increasing use of expert pre-operative planning and patient analysis services by the UNiD Lab™ engineering teams using the proprietary UNiD™ platform represents a significant revenue potential for Medicrea due to two opportunities:
- UNiD™ patient-specific rods are systematically implanted with associated implants (screws, connectors) marketed by the Company. This is currently the case for only 50% of UNiD™ surgeries in the United States due to surgeons implanting "Tulip-based” pedicle screw systems from their historical suppliers in combination with Medicrea's UNiD™ patient-specific rods. A steady increase of this ratio will allow Medicrea to significantly increase its revenue in this market, especially since the Company now has a product portfolio that adapts to all surgeons’ practices, particularly since the FDA approval of its new Tulip Genesis screw system, whose first kits have just been delivered in the United States for its commercial launch in Q3 2019. This recent approval from the FDA will be a key growth driver for the Company and will be announced in detail within the next few days.
- In order to enhance innovation and accelerate the monetization of the expert services powered by the predictive model and Artificial Intelligence developed on the clinical database collected by the UNiD ASI™ platform, Medicrea also plans to invoice UNiD™ patient-specific rod planning services in the USA, and not just the implants that result from surgical planning.
“We have developed an unparalleled surgical planning software platform that positions us as a unique player in personalized spine surgery. Thanks to the UNiD ASI™ technology and the skills of our UNiD Lab™ engineers, we dramatically improve the standard of care of spinal pathologies by offering a solution adapted to each patient. This offer brings significant added value to our customers. We will continue to deploy capital expenditures and resources to accelerate the adoption of this technology”, commented Denys Sournac, President and CEO of Medicrea.
Next publication: Results for the First Half of 2019: Wednesday, September 18, 2019, after-market.
About Medicrea (www.medicrea.com)
Through the lens of predictive medicine, Medicrea leverages its proprietary software analysis tools with big data and machine learning technologies supported by an expansive collection of clinical and scientific data. The Company is well-placed to streamline the efficiency of spinal care, reduce procedural complications and limit time spent in the operating room.
Operating in a $10 billion marketplace, Medicrea is a Small and Medium sized Enterprise (SME) with 200 employees worldwide, which includes 50 who are based in the U.S. The Company has an ultra-modern manufacturing facility in Lyon, France housing the development and production of 3D- printed titanium patient-specific implants.
For further information, please visit: Medicrea.com.
Connect with Medicrea
FACEBOOK | INSTAGRAM | TWITTER | WEBSITE | YOUTUBE
Medicrea is listed on
EURONEXT Growth Paris
ISIN: FR 0004178572
Ticker: ALMED
LEI: 969500BR1CPTYMTJBA37
Medicrea is traded on
OTCQX Best Market
Ticker: MRNTF