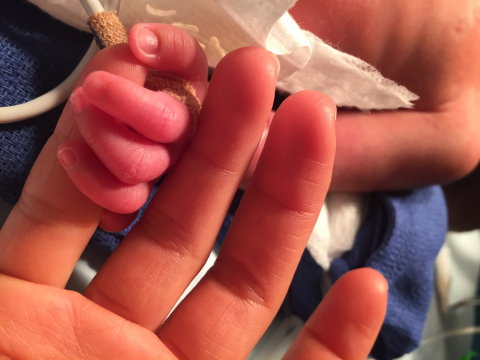
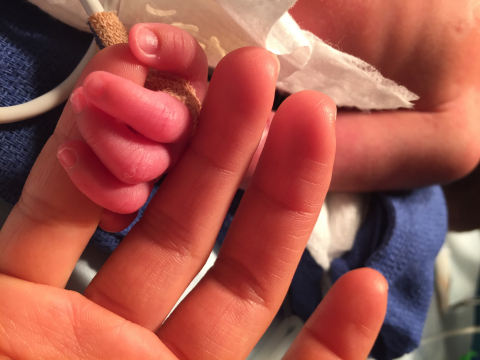
ST. PAUL, Minn.--(BUSINESS WIRE)--St. Jude Medical, Inc. (NYSE:STJ), a global medical device company, today announced the launch of the ADO II AS (AMPLATZER™ Duct Occluder II Additional Sizes) pediatric clinical trial. The U.S. IDE clinical trial will evaluate the safety and effectiveness of the St. Jude Medical™ AMPLATZER™ Duct Occluder II AS (ADO II AS), a first-of-its-kind device specifically designed for closure of the small patent ductus arteriosus (PDA). St. Jude Medical now has two ongoing U.S. cardiology IDE clinical trials for pediatric patients. In 2014, the company launched the HALO trial to evaluate the safety and effectiveness of the SJM™ Masters HP Series 15mm mechanical heart valve, the smallest pediatric mechanical heart valve in the world.
Congenital heart disease (CHD) is the most common cause of major congenital anomalies, representing a major global health challenge. Every year CHD affects 1.35 million babies worldwide. Nearly 25 percent of babies with a congenital heart defect require surgery during their first year.
“The patients who would be eligible for this study are the tiniest and most fragile we care for—severely premature newborns who in many cases are critically ill from the presence of a patent ductus arteriosus (PDA),” said national investigator Dr. Evan Zahn, director of the Guerin Family Congenital Heart Program in the Cedars-Sinai Heart Institute and Department of Pediatrics at Cedars‐Sinai Medical Center in Los Angeles. “The AMPLATZER Duct Occluder II AS will provide an experimental option to surgery. Surgery has many risks in this delicate population and a minimally invasive approach is desperately needed for this fairly common and often quiet serious problem.”
Despite the large number of babies worldwide born with CHD, many children are treated with adult-sized devices, which may not be optimal for their tiny hearts. St. Jude Medical and the U.S. Food & Drug Administration (FDA) have long recognized the need for solutions and educational resources to support pediatric patients.
In 2007, the U.S. Congress passed the Pediatric Medical Device Safety and Improvement Act to prompt development of medical devices designed and engineered specifically for the pediatric patient population. In collaboration with the FDA and top congenital heart disease experts, St. Jude Medical is addressing the unique challenges of developing, evaluating and bringing to market devices intended for pediatric use.
With more than 17 years of demonstrated clinical experience, the AMPLATZER™ family of occluders is the most studied transcatheter cardiac defect closure devices in the marketplace today. Adding to this body of clinical data, specifically for pediatrics, the ADO II AS IDE trial will enroll a maximum of 50 patients at up to 10 sites nationwide. Patients in the trial will fall within two study groups:
- A minimum of 15 patients less than or equal to 2 KG (approx. 4.4 pounds)
- A minimum of 25 patients greater than 2 KG
Designed to treat PDA—one of the most common of all congenital heart anomalies—the ADO II AS device conforms to the smallest ducts while achieving complete closure from a pulmonary or aortic artery approach. Data collected during the ADO II AS trial across all trial sites will be used to support a pre-market approval submission of the device to FDA. The ADO II AS device is currently approved for use in more than 50 countries outside the United States.
“St. Jude Medical is continuing to build its legacy of innovation in the field of pediatric cardiology, and working to break the barriers to developing new CHD treatments so more children’s health conditions can be properly addressed,” said Dr. Srijoy Mahapatra, vice president of clinical, medical and scientific affairs at St. Jude Medical. “We are passionate about helping kids thrive, along with creating medical solutions that save and improve their lives.”
Building on the Momentum of the HALO IDE Clinical Trial
The
other pediatric clinical trial from St. Jude Medical is evaluating the
safety and efficacy of the SJM Masters HP Series 15mm mechanical heart
valve, the smallest pediatric mechanical heart valve in the world. In
the United States alone, more than 35,000
babies are born each year with congenital heart defects, some of
which will impact valve function to the point they cannot be repaired
and will require surgical
valve replacement. The HALO
IDE trial is enrolling pediatric patients in need of mitral valve
replacement who cannot be implanted with the current range of approved
valves without additional risks and who have no alternative approved
treatment options.
The HALO IDE trial stems from a new approach to the design, evaluation and regulatory approval of pediatric heart valves that emerged from a FDA-led workshop in 2010 organized in response to the pressing need to develop products for this patient population. During the 2nd Annual Pediatric Surgical Innovation Symposium, former FDA Commissioner Dr. Margaret A. Hamburg cited the St. Jude Medical pediatric heart valve as an example of what collaboration can achieve when industry, FDA staff, clinicians and academics come together to support pediatric medical device development.
About We Heart Kids™ Initiative from St. Jude Medical
At St.
Jude Medical, we are inspired by kids living with congenital heart
disease and their heroic families who never give up hope. That is one
reason we are proud to be a market leader in the research, design and
development of electrophysiology and interventional cardiology solutions
for pediatric patients worldwide. Through the We Heart Kids initiative,
St. Jude Medical partners with patient advocacy groups globally to help
provide information and access to high-quality pediatric care in
underserved communities. To learn more about our We Heart Kids
initiative and our commitment to pediatric cardiology, please visit sjm.com/weheartkids.
About St. Jude Medical
St. Jude Medical is a leading global
medical device manufacturer and is dedicated to transforming the
treatment of some of the world's most expensive epidemic diseases. The
company does this by developing cost-effective medical technologies that
save and improve lives of patients around the world. Headquartered in
St. Paul, Minn., St. Jude Medical employs approximately 18,000 people
worldwide and has five major areas of focus that include heart failure,
atrial fibrillation, neuromodulation, traditional cardiac rhythm
management and cardiovascular. For more information, please visit sjm.com
or follow us on Twitter @SJM_Media.
Forward-Looking Statements
This news release contains
forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995 that involve risks and uncertainties. Such
forward-looking statements include the expectations, plans and prospects
for the company, including potential clinical successes, reimbursement
strategies, anticipated regulatory approvals and future product
launches, and projected revenues, margins, earnings and market shares.
The statements made by the company are based upon management’s current
expectations and are subject to certain risks and uncertainties that
could cause actual results to differ materially from those described in
the forward-looking statements. These risks and uncertainties include
market conditions and other factors beyond the company’s control and the
risk factors and other cautionary statements described in the company’s
filings with the SEC, including those described in the Risk Factors and
Cautionary Statements sections of the company’s Annual Report on Form
10-K for the fiscal year ended January 2, 2016 and Quarterly Report on
Form 10-Q for the fiscal quarter ended July 2, 2016. The company does
not intend to update these statements and undertakes no duty to any
person to provide any such update under any circumstance.