ORLANDO, Fla.--(BUSINESS WIRE)--Xēnix Medical, a surgical implant company focused on the development of novel science-based solutions for patients requiring spinal fusion surgery, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for nanotechnology designation of its neoWave™ line of interbody fusion implants featuring proprietary NANOACTIV™ surface technology.
The nanotechnology designation enhances claims for the neoWave cervical and lumbar interbody devices with NANOACTIV surface, of which over 7,000 have been implanted in the United States.
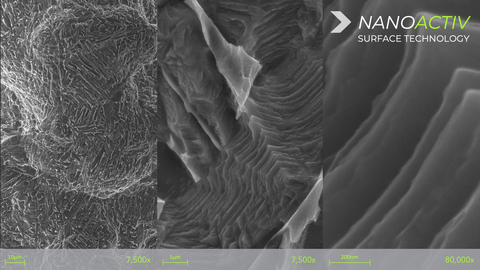
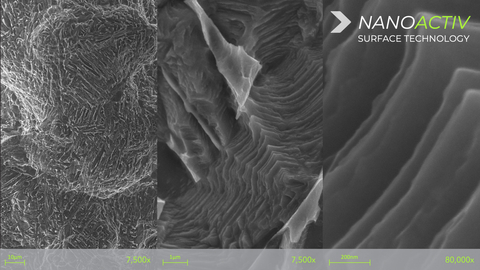
The NANOACTIV micro and nano-roughened surface is designed to improve fixation to adjacent bone, and has been engineered with nano-scale surface features at a nanometer (10-9) level, which has demonstrated the ability to elicit an endogenous cellular and biochemical response as represented by differentiation of human mesenchymal stem cells through the osteogenic lineage and production of a mineralized matrix in vitro**, as compared to an untreated surface*. The NANOACTIV implant surface demonstrates elements to be considered a nanotechnology as outlined in FDA nanotechnology guidance document.
“Compared to previous generation interbody technologies I have used in practice, I have found that the neoWave implants provide the best opportunity to achieve a robust arthrodesis,” says Robert Hirschl, MD, Founder and Neurosurgeon in Orlando, Florida. “My clinical results using these implants lead me to believe that the unique fully porous implant, large endplate contact area and nano scale surface are key to helping patients achieve optimal clinical outcomes, while reducing the incidence of subsidence and maintaining favorable imaging characteristics. The FDA’s acknowledgement of the NANOACTIV nanoscale surface benefit versus a standard 3D printed surface further supports my experience and is another distinguishing feature that advances these implants into a class of its own.”
Justin Brown, Ph.D., Associate Professor of Biomedical Engineering at The Pennsylvania State University said, “Our evaluation of the NANOACTIV surface technology revealed that the micro- and nanoscale features generated a bioinstructive interface that promoted robust osteogenic differentiation of mesenchymal stem cells. It is exciting to see advancements in Ti6Al4V surface technology that parallel the bioinstructive features we strive to engineer into biomaterial scaffolds. In my opinion, the NANOACTIV surface technology has potential to dramatically improve patient outcomes.”
The structurally advanced neoWave interbody technology consists of a patented 3D-printed lattice design that retains strength and reduced device stiffness without the use of traditional implant framework or solid walls. The neoWave matrix has been shown to increase endplate contact surface area and resistance to subsidence compared to a similar device containing solid framework and/or traditional anti-migration teeth*.
Ryan Phillips, President of Xēnix Medical, commented, “Receiving nanotechnology clearance for the neoWave interbody systems is an incredible milestone and achievement for Xēnix, elevating the neoWave implant systems into a distinct device category shared only by a few companies in the industry. As we actively develop a complete line of neoWave interbody devices, the NANOACTIV surface will be a dominant enhancement of future implant systems.
The unique combination of a nanotechnology surface and completely latticed implant presents a significant breakthrough in treating patients that require interbody fusion. We believe this designation evidences the potential benefit of nanotechnology to patient outcomes and improving care.
This clearance is testament to our amazing team who have spent years in the development and research of NANOACTIV.”
*Data on file.
**In vitro performance may not be representative of clinical performance.
About Xēnix Medical
Xēnix Medical is a surgical implant company focused on the development of novel solutions to advance the science of fusion engineering to enhance the treatment of various pathologies for patients requiring spinal fusion surgery. The company, headquartered in Orlando, Florida, is in active development of multiple unique implant technology platforms and markets its line of spinal implant devices in the United States through a network of independent distributors. To learn more, visit xenixmedical.com.