SpineX Demonstrates Groundbreaking Technology to Treat Children with Cerebral Palsy
SpineX Demonstrates Groundbreaking Technology to Treat Children with Cerebral Palsy
SpineX Publishes Results from First in Human Study Using its Proprietary Non-Surgical and Non-Invasive Device SCiP to Treat children with Cerebral Palsy
LOS ANGELES--(BUSINESS WIRE)--SpineX, Inc., a clinical stage medtech company today announced the groundbreaking results its first in human study in children with cerebral palsy. The study is published in the esteemed medical journal Nature Communications demonstrating unparalleled functional improvements with its proprietary non-surgical treatment SCiPTM (Spinal Cord Innovation in Pediatrics) in children with Cerebral Palsy (CP). “A pilot study combining noninvasive spinal neuromodulation and activity-based neurorehabilitation therapy in children with cerebral palsy”, led by Dr. Susan Hastings, PT, DPT and Dr. V Reggie Edgerton, PhD is the result of years of work championed by the two stalwarts of their respective fields. This study discusses how delivering non-invasive spinal neuromodulation, using SCiPTM, during Physical Therapy improved voluntary sensorimotor function in 16 out of 16 children over a wide range of ages and severities of CP. SpineX was awarded the Breakthrough Device Designation (BDD) from US FDA for SCiP and the proposed treatment of CP. In addition, SpineX has engaged with FDA to gain alignment on a proposed clinical trial to be conducted in 2023; the results of which are anticipated to lead to FDA clearance of the SCiPTM device for the treatment of CP.
CP is severely debilitating with no current cure. Over 10,000 new cases are diagnosed each year where CP is the result of damage to the developing brain and describes a group of movement disorders that affect a person’s ability to move and maintain balance and posture. To date there are no methods or medications to prevent or treat CP. Often children with CP are left to be treated with invasive surgeries that attempt to decrease spasticity, a common symptom of the disease.
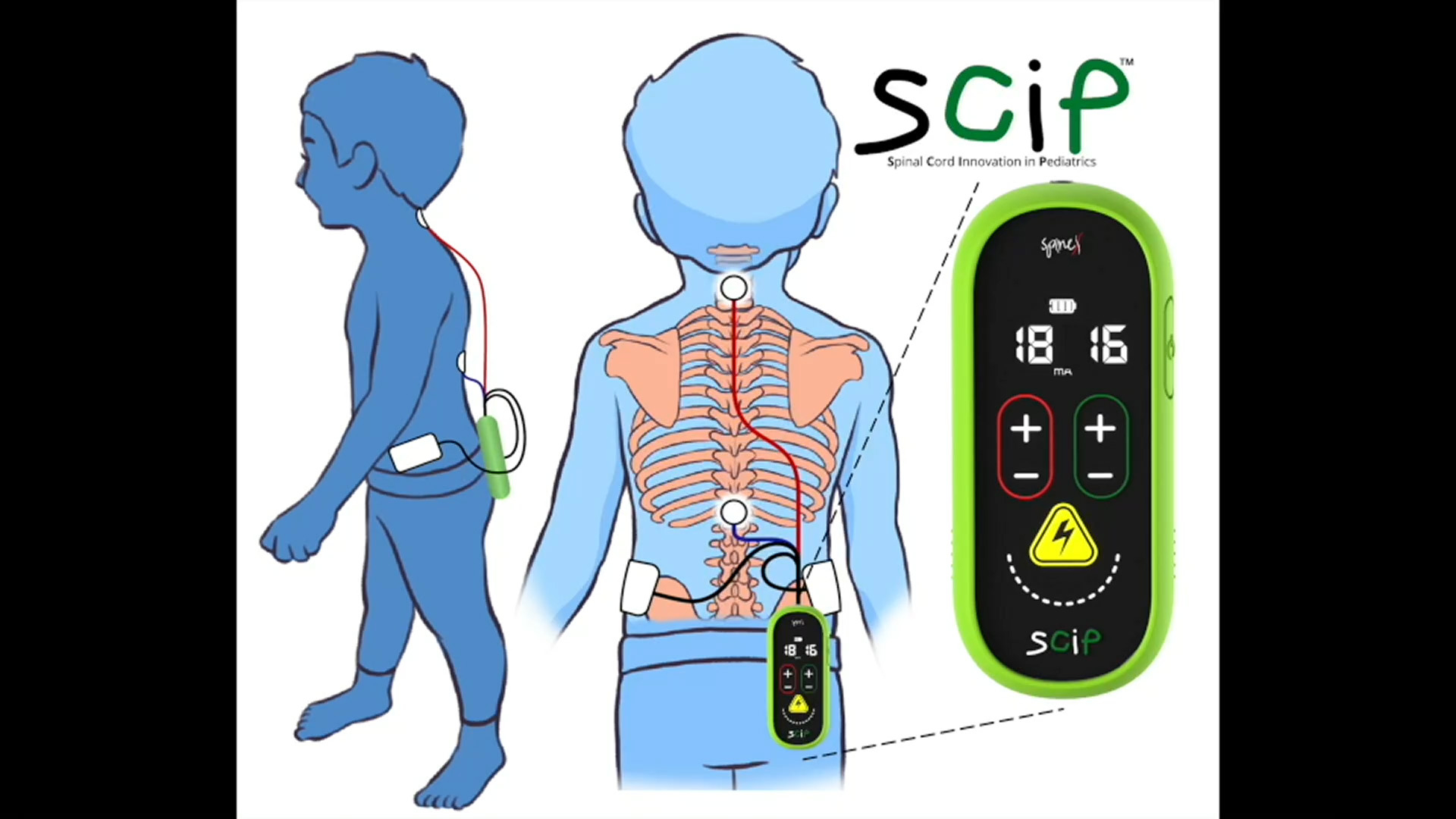
SCiPTM is a non-invasive spinal electrical neuromodulation device which provides transcutaneous spinal cord neuro-stimulation to potentially treat the underlying neurological dysfunction in pediatric patients with CP. SCiPTM aims to be the first medical device in the U.S. to treat CP by transforming the brain and spinal cord dysfunctional connectivity into highly functional systems.
“At just three years old and highly affected by CP, our son has shown such exciting progress since he began using SCiP,” said Dana, the mother of a young boy diagnosed with CP who was enrolled in a clinical study sponsored by SpineX. “We’re so grateful that our son was included in the clinical study with SCiPTM as it has opened up a world of possibilities for our son, and we hope others will be able to witness it soon.”
Spinal Neuromodulation is being developed as a platform technology by SpineX and SCiPTM represents their second Breakthrough Device Designation following the SCONETM device which is in clinical trials for the treatment of Neurogenic Bladder.
About SpineX Inc.
SpineX Inc. is a clinical stage bioelectric medtech company developing noninvasive neuromodulation devices. SCiPTM by SpineX Inc. is an investigational device being developed to treat CP and is limited by Federal (USA) law to investigational use only. FDA has not yet reviewed the safety or effectiveness of SCiP. SpineX is planning to submit a marketing submission for FDA review and clearance. To learn more about SpineX, visit www.spinex.co or follow @spinex_inc on social media.
Contacts
Parag Gad
parag@spinex.co
408 203 5061