BOSTON--(BUSINESS WIRE)--PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) ("PureTech" or the "Company"), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced the achievement of proof-of-principle for its Glyph™ platform in a healthy adult study of LYT-300 (oral allopregnanolone), PureTech’s wholly-owned therapeutic candidate for the potential treatment of a range of neurological and neuropsychological conditions. This is a key milestone for the candidate, which is designed to overcome the normally poor oral bioavailability of allopregnanolone to deliver its proven efficacy via simple, convenient oral dosing. This is also the first mechanistic proof-of-principle in the clinic for PureTech’s Glyph lymphatic targeting platform, which is designed to bypass first-pass metabolism to help maximize the therapeutic potential of validated targets and drugs where oral bioavailability has been a barrier. The Glyph platform is also designed to have additional applications through its ability to selectively traffic therapeutics into the lymphatic system, potentially enabling more direct targeting of the immune system.
“Natural allopregnanolone has demonstrated efficacy for the treatment of postpartum depression (PPD) and other neuropsychological conditions, but up to now has required IV delivery due to high first-pass liver metabolism. LYT-300 is designed to unlock the validated pharmacology of natural allopregnanolone with a potential oral treatment option for PPD and a range of other neurological and neuropsychological conditions,” said Julie Krop, M.D., Chief Medical Officer of PureTech. “Achieving significant systemic exposure of allopregnanolone with orally administered LYT-300 is also a key validation for our Glyph lymphatic targeting platform and represents proof-of-principle for being able to administer drugs orally that currently require IV administration due to first-pass liver metabolism.”
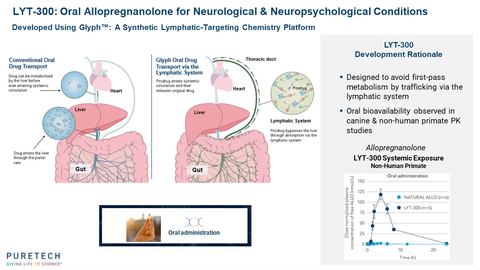
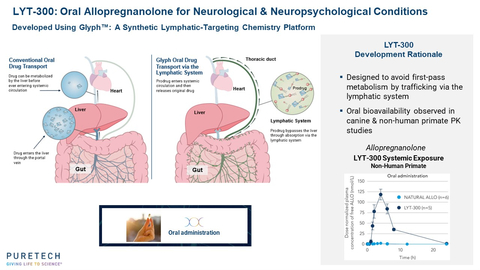
PureTech has previously presented data in non-human primates with LYT-300 demonstrating significantly greater oral bioavailability than orally administered allopregnanolone. Data from this Phase 1 program reaffirmed this finding in humans, showing bioavailability of allopregnanolone that was approximately nine-fold greater than that of orally administered allopregnanolone, based on previously published data.1 In the PureTech study, fasted healthy adults were given LYT-300 containing the equivalent of 53 mg of allopregnanolone, achieving plasma exposure levels with an AUCinf of 352 ng*hr/mL. This represents a nine-fold increase in dose-adjusted exposure when compared to a previously published study in fasted healthy adults in which 30 mg of allopregnanolone was orally dosed, resulting in an AUCinf of 21 ng*hr/mL.1
Allopregnanolone is a natural neurosteroid with well-established biology that has demonstrated efficacy for the treatment of epilepsy, depression and other neurological indications, but its poor oral bioavailability has limited its development as a therapeutic. The United States Food and Drug Administration (FDA) has approved a 60-hour intravenous infusion formulation of allopregnanolone for the treatment of PPD, though this method of administration has inherent limitations. The Glyph platform is designed to leverage the body’s natural lipid absorption and transport process to overcome these limitations to enable oral administration. Demonstrating oral bioavailability with the Glyph platform opens up the possibility of developing other natural neurosteroids and a number of bioactive molecules as oral therapies to treat a range of serious diseases.
“Due to a relative paucity of newly FDA-approved molecules in recent decades, significant patient needs exist, and we are experiencing a national mental health crisis that makes these needs even greater. Achieving an orally bioavailable prodrug of allopregnanolone may prove promising in the treatment of mood disorders such as postpartum depression – and potentially a range of related neurological and neuropsychiatric conditions,” said Maurizio Fava, M.D., Psychiatrist-in-Chief at Massachusetts General Hospital.
“Given the proven efficacy of allopregnanolone in postpartum depression and its potential in a range of other conditions, significant effort has been put into delivering oral formulations for the molecule, without success. This has led to synthetic approaches that may not capture the full therapeutic potential of natural allopregnanolone,” said Scott Reines, M.D., Ph.D., former Senior Vice President, J&J Pharmaceutical Research & Development. “I am excited by the data generated with PureTech’s LYT-300 and believe it could represent an exciting step towards enabling bioavailability for this and other classes of therapeutics.”
The multi-part Phase 1 program of LYT-300 has three primary objectives – to demonstrate oral bioavailability, evaluate safety and tolerability across a range of doses, and to identify a dose to take forward. With the achievement of the first objective, additional dose exploration and the effect of food on oral absorption of the prodrug are progressing, and assessments of safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) will be measured. Dose escalation continues as no dose-limiting toxicities have been observed to date.
As allopregnanolone is a modulator of the GABAA receptor, the Phase 1 program will also explore the impact of LYT-300 on b-EEG, and other markers of GABAA target engagement, thus potentially providing further early insights into the mechanistic effects of LYT-300 and its potential in a range of indications where GABAA receptors play a key biological role. Completion of the rest of the Phase 1 program is expected by the end of 2022, and – if data are favorable – a Phase 1b/2a study is planned to initiate in 2023.
Results from the Phase 1 program will be shared in future scientific forums.
About LYT-300
LYT-300 is a clinical therapeutic candidate that is in development as a potential treatment for a range of neurological and neuropsychological conditions. Developed using PureTech's Glyph technology platform, LYT-300 is an oral prodrug of natural allopregnanolone. An IV formulation of allopregnanolone is approved by the United States Food and Drug Administration and administered as a 60-hour infusion for the treatment of postpartum depression. Allopregnanolone is a positive allosteric modulator of γ-aminobutyric-acid type A (GABAA) receptors and has been shown to regulate mood and other neurological conditions. PureTech initiated a Phase 1 clinical study of LYT-300 in late 2021, which is designed to characterize the safety, tolerability, PK and PD of orally administered LYT-300 in healthy volunteers. Allopregnanolone is a neuroactive steroid and positive allosteric modulator (PAM) of GABAA receptors. Unlike benzodiazepines, allopregnanolone can provide both transient and longer-term normalization of overactive neural circuits because it also acts at GABA receptors outside of synapses.2 Dual intra- and extra-synaptic GABA PAMs have been shown to not only improve sleep,3 but also mood.1
About the Glyph™ Technology Platform
Glyph is PureTech's synthetic lymphatic-targeting chemistry platform which is designed to employ the lymphatic system's natural lipid absorption and transport process to enable the oral administration of therapeutics. Glyph reversibly links a drug to a dietary fat molecule, creating a novel prodrug. The linked fat molecule re-routes the drug's normal path to the systemic circulation, bypassing the liver and instead moving from the gut into the lymphatic vessels that normally process dietary fats. PureTech believes this technology has the potential to (1) enable direct modulation of the immune system via drug targets present in mesenteric lymph nodes and (2) provide a broadly applicable means of enhancing the bioavailability of orally administered drugs that would otherwise be reduced by first-pass liver metabolism. PureTech is leveraging validated biology to accelerate the development of a Glyph portfolio, prioritizing highly characterized drugs to enhance with the Glyph technology based on the potential value unlocked in improving their oral bioavailability or lymphatic targeting. Glyph has achieved mechanistic proof-of-principle in the clinic with the first Glyph-generated therapeutic candidate, LYT-300 (oral allopregnanolone), which is being evaluated in a Phase 1 study. PureTech has exclusively licensed the Glyph technology platform, which is based on the pioneering research of Christopher Porter, Ph.D., and his research group at the Monash Institute of Pharmaceutical Sciences at Monash University. The Porter Research Group and collaborators have published research in Nature Metabolism, Frontiers in Pharmacology and the Journal of Controlled Release supporting the Glyph platform's ability to directly target the lymphatic system with a variety of therapies.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including inflammatory, fibrotic and immunological conditions, intractable cancers, lymphatic and gastrointestinal diseases and neurological and neuropsychological disorders, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders.
This pipeline, which is being advanced both internally and through PureTech's Founded Entities, is comprised of 27 therapeutics and therapeutic candidates, including two that have received both U.S. FDA clearance and European marketing authorization, as of the date of PureTech's most recently filed Annual Report and corresponding Form 6-K. All of the underlying programs and platforms that resulted in this pipeline of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on unique insights in immunology and drug development.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including those related to the design and applicability of the Glyph lymphatic targeting platform, including other potential development options and applications of the platform, the potential treatments associated with LYT-300, our LYT-300 clinical development plans and programs, including our Phase 1 program and expected timing associated therewith, and our future prospects, developments and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks, uncertainties and other important factors that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks, uncertainties and other important factors described under the caption "Risk Factors" in our Annual Report on Form 20-F for the year ended December 31, 2021 filed with the SEC and in our other regulatory filings. These forward-looking statements are based on assumptions regarding the present and future business strategies of the Company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, we disclaim any obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
1 Brexanolone NDA 211371 Multi-disciplinary Review and Evaluation, FDA CDER, 2018.
2 Ghit, A., Assal, D., Al-Shami, A.S. Hussein D.E.E. GABAA receptors: structure, function, pharmacology, and related disorders. J Genet Eng Biotechnol 19, 123 (2021). https://doi.org/10.1186/s43141-021-00224-0
3 Bullock, A., Kaul, I., Li, S., Silber, C., Doherty, J., & Kanes, S. J. (2021). Zuranolone as an oral adjunct to treatment of Parkinsonian tremor: A phase 2, open-label study. Journal of the neurological sciences, 421, 117277. https://doi.org/10.1016/j.jns.2020.117277