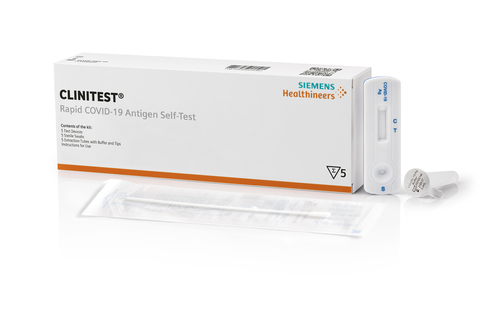
TARRYTOWN, N.Y.--(BUSINESS WIRE)--Siemens Healthineers announces that the U.S. Food and Drug Administration (FDA) today granted Emergency Use Authorization (EUA) for the CLINITEST Rapid COVID-19 Antigen Self-Test,1, 2, 3 providing nationwide access to a new at-home or over-the-counter self-test as COVID-19 testing needs continue to grow for individuals, families, and businesses. The easy-to-use nasal swab test is intended to aid in the rapid detection of SARS-CoV-2 (the virus that causes COVID-19) and provides visually read test results in just 15 minutes. It is authorized for self-testing use by individuals age 14 and older or adult-collected samples from individuals ages 2 to 13 years. The test is expected to be available starting in January. Siemens Healthineers has secured dedicated production capacity for U.S. bound product in the tens of millions per month.
“Undetected COVID-19 exposure is a major driver of community spread. With the CLINITEST Rapid COVID-19 Antigen Self-Test, consumers will have access to a fast, reliable, and convenient test to provide additional safety,” said Christoph Pedain, PhD, Head of Point of Care Diagnostics, Siemens Healthineers. “Siemens Healthineers is proud to bring this high-quality test, already used and trusted by families, medical professionals, businesses and governments in many parts of the world, to the United States under FDA Emergency Use Authorization.”
The product comes in a specific configuration for the United States and has been evaluated in a study specifically designed for the U.S. market and fully conducted in the U.S. with laypersons, thereby covering currently circulating variants of SARS-CoV-2. Meeting the high threshold of the US FDA, the product is highly accurate, with sensitivity of 86.5% (95% CI: 79.6 to 91.3%) and a specificity of 99.3% (95% CI: 95.9 to 100.0%) compared to the nucleic acid (PCR) detection method. Sensitivity defines the test’s ability to produce a positive result in those infected with SARS-CoV-2 per the PCR reference method, and specificity defines the ability to produce a negative result in those not currently infected with SARS-CoV-2.
All clinical data has been established under Siemens Healthineers participation in the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics’ (RADx) Independent Test Assessment Program (ITAP).
“The CLINITEST Rapid COVID-19 Antigen Self-Test detects SARS-CoV-2 antigens in people who are actively infected with the virus, making important information more readily available,” added Dr. Pedain. “We’d like to thank the U.S. Government, specifically the National Institutes of Health Rapid Acceleration of Diagnostics’ Independent Test Assessment Program, for their partnership in bringing much needed tests to the American people. We know this is a critical time in this pandemic, and we will do our part in providing support.”
Reliable results can help consumers make confident, informed decisions about their daily lives, with the simplicity of a nasal swab test at home. Consumers who receive a positive test result should follow guidance from the Centers for Disease Control and Prevention (CDC) to isolate and take steps to mitigate the spread of COVID-19.
Siemens Healthineers response to the pandemic
Siemens Healthineers has distinguished itself as a provider of quality diagnostic assays to aid the fight against the COVID-19 pandemic. In addition to antibody, antigen, and molecular SARS-CoV-2 tests, Siemens Healthineers offers a broad diagnostics portfolio across point of care and laboratory applications to aid in the prognosis, treatment and follow-up of COVID-19 patients. The company’s broad and differentiated menu includes hematology, coagulation, cardiac, respiratory, inflammation and infectious disease panels. Blood gas testing and imaging solutions from Siemens Healthineers deliver actionable results that aid clinicians in caring for COVID-19 patients.
For further information on the CLINITEST Rapid COVID-19 Antigen Self-Test, please visit www.clinitest.siemens-healthineers.com/US.
1 Product availability varies by country. Distributed by Siemens Healthineers.
2 The FDA’s EUA decision is based on the company's participation in the NIH Rapid Acceleration of Diagnostics’ (RADx) Independent Test Assessment Program (ITAP), which aims to accelerate the regulatory review and availability of high-quality, accurate and reliable OTC test to the American public.
3 In the USA, this product has not been FDA cleared or approved; but has been authorized by FDA under an Emergency Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Siemens Healthineers AG (listed in Frankfurt, Germany: SHL) pioneers breakthroughs in healthcare. For everyone. Everywhere. As a leading medical technology company headquartered in Erlangen, Germany, Siemens Healthineers and its regional companies is continuously developing its product and service portfolio, with AI-supported applications and digital offerings that play an increasingly important role in the next generation of medical technology. These new applications will enhance the company’s foundation in in-vitro diagnostics, image-guided therapy, in-vivo diagnostics, and innovative cancer care. Siemens Healthineers also provides a range of services and solutions to enhance healthcare providers’ ability to provide high-quality, efficient care. In fiscal 2021, which ended on September 30, 2021, Siemens Healthineers, which has approximately 66,000 employees worldwide, generated revenue of €18.0 billion and adjusted EBIT of €3.1 billion. Further information is available at www.siemens-healthineers.com.