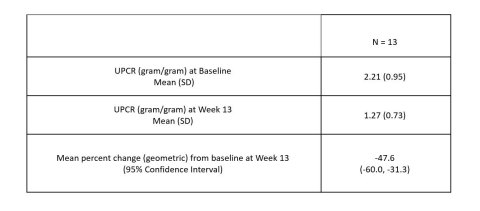
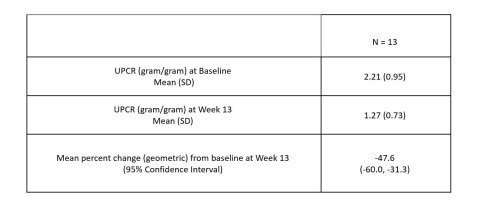
BOSTON--(BUSINESS WIRE)--Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that, in a Phase 2 proof-of-concept (POC) study in patients with APOL1-mediated focal segmental glomerulosclerosis (FSGS), VX-147 on top of standard of care achieved a statistically significant, substantial and clinically meaningful mean reduction of 47.6% in the urine protein to creatinine ratio (UPCR) at Week 13 compared to baseline. VX-147 was well tolerated. These results provide the first clinical evidence and POC that an oral small molecule APOL1 inhibitor can decrease proteinuria in patients with APOL1-mediated kidney disease. Based on these results, Vertex plans to advance VX-147 into pivotal development in APOL1-mediated kidney disease, including FSGS, in Q1 2022.
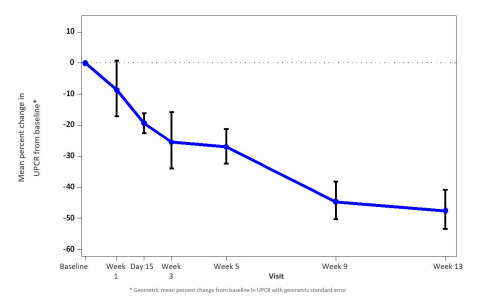
A total of 16 patients were enrolled in the study. According to the pre-specified statistical analysis plan, three patients who were noncompliant with treatment were not included in the primary efficacy analysis. In the 13 evaluable patients, treatment with VX-147 on top of standard of care resulted in a rapid, statistically significant and clinically meaningful mean change in proteinuria from baseline of -47.6% (95% CI: -60.0%, -31.3%) following 13 weeks of treatment. Reduction in proteinuria was observed early and continued throughout the 13-week treatment period. Results were consistent regardless of baseline proteinuria or background therapy.
There were no treatment discontinuations due to adverse events (AEs), and there were no serious adverse events considered related to study drug. All AEs were mild or moderate in severity. The most common AEs (occurring in > 15% of subjects) were headache, back pain and nausea.
“VX-147 is the first investigational treatment targeting the underlying cause of APOL1-mediated kidney disease. These results demonstrate that inhibition of the APOL1 protein can substantially reduce proteinuria in patients with two APOL1 genetic variants, FSGS and significant proteinuria,” said Carmen Bozic, M.D., Executive Vice President, Global Medicines Development and Medical Affairs, and Chief Medical Officer at Vertex. “We are working with urgency to advance this molecule into pivotal development with the goal of bringing this first-in-class therapy to the more than 100,000 patients in the U.S. and Europe living with APOL1-mediated kidney disease.”
“These results are very promising. For decades, I have cared for patients who have suffered from the rapid onset of kidney disease that progresses quickly to kidney failure, which we now know may have been caused by APOL1 gene variants,” said Glenn Chertow, M.D., M.P.H., Professor of Medicine, Stanford University School of Medicine, and Chair of the Vertex APOL1 Program Steering Committee. “This approach has tremendous potential, as it targets the underlying genetic driver of kidney disease in these patients. These data demonstrate the potential of VX-147 as a targeted treatment for a patient population at unusually high risk of progression to kidney failure.”
Based on these data, Vertex plans to advance VX-147 into pivotal development in Q1 2022 as a potential first-in-class therapy for the more than 100,000 patients with proteinuric kidney disease mediated by variants in the APOL1 gene, including, but not limited to, FSGS. Consistent with the company’s serial innovation strategy, Vertex will also continue to investigate additional small molecule APOL1 inhibitors in the clinic.
About the VX-147 Phase 2 Study
The Phase 2 open-label, single-arm study evaluated the efficacy, safety and pharmacokinetics of VX-147 in patients with APOL1-mediated FSGS. Patients with biopsy-confirmed FSGS, two APOL1 genetic variants, proteinuria defined by at least 0.7 g/g in the UPCR and an estimated glomerular filtration rate (eGFR) of at least 27 mL/min/1.73 m2 were eligible for enrollment in the study. Patients were on a stable standard-of-care regimen, which could include an angiotensin-converting enzyme (ACE) inhibitor, an angiotensin II receptor blocker (ARB), immunosuppressants and/or low doses of corticosteroids. Patients were treated with VX-147 for a total of 13 weeks. The primary endpoint was percent change from baseline in UPCR at Week 13. The secondary endpoints were safety and pharmacokinetics. In addition, there is a 28-day safety follow-up period after the last dose of treatment and an optional off-treatment follow-up period of up to 12 weeks after the last dose of treatment. The study is ongoing for these follow-up periods.
About APOL1-Mediated Kidney Disease
APOL1-mediated kidney disease is a form of chronic kidney disease caused by variants of the APOL1 gene. Over 100,000 people in the U.S. and Europe have two APOL1 genetic variants and proteinuric kidney disease. People who inherit two risk variants in the APOL1 gene have a course of disease that is far more aggressive than in the absence of APOL1 genetic variants. Inherited APOL1 genetic variants cause kidney disease through a toxic gain of function, which leads to podocyte injury. This injury disrupts filtration, resulting in proteinuria and rapidly progressive kidney disease. Progressive kidney disease can result in dialysis, kidney transplant or death.
Conference Call and Webcast
The company will host a conference call and webcast today at 8:30 am ET. To access the call, please dial (866) 501-1537 (U.S.) or +1 (720) 545-0001 (International). The conference call will be webcast live and a link to the webcast can be accessed through Vertex's website at www.vrtx.com in the "Investors" section under "Events and Presentations." To ensure a timely connection, it is recommended that users register at least 15 minutes prior to the scheduled webcast. An archived webcast will be available on the company's website.
About Vertex
Vertex is a global biotechnology company that invests in scientific innovation to create transformative medicines for people with serious diseases. The company has multiple approved medicines that treat the underlying cause of cystic fibrosis (CF) — a rare, life-threatening genetic disease — and has several ongoing clinical and research programs in CF. Beyond CF, Vertex has a robust pipeline of investigational small molecule medicines in other serious diseases where it has deep insight into causal human biology, including pain, alpha-1 antitrypsin deficiency and APOL1-mediated kidney disease. In addition, Vertex has a rapidly expanding pipeline of cell and genetic therapies for diseases such as sickle cell disease, beta thalassemia, Duchenne muscular dystrophy and type 1 diabetes mellitus.
Founded in 1989 in Cambridge, Mass., Vertex's global headquarters is now located in Boston's Innovation District and its international headquarters is in London. Additionally, the company has research and development sites and commercial offices in North America, Europe, Australia and Latin America. Vertex is consistently recognized as one of the industry's top places to work, including 12 consecutive years on Science magazine's Top Employers list, one of the 2021 Seramount (formerly Working Mother Media) 100 Best Companies, and a best place to work for LGBTQ equality by the Human Rights Campaign. For company updates and to learn more about Vertex's history of innovation, visit www.vrtx.com or follow us on Facebook, Twitter, LinkedIn, YouTube and Instagram.
Special Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, (i) statements by Dr. Carmen Bozic and Dr. Glenn Chertow in this press release, (ii) our plans to advance VX-147 into pivotal development in Q1 2022, (iii) our expectations for the potential benefits of VX-147 as a treatment for patients with APOL1-mediated kidney disease, (iv) our expectation that VX-147 is a potential first-in-class therapy for more than 100,000 patients living with APOL1- mediated kidney disease, and (v) statements regarding our plans to investigate additional small molecule APOL1 inhibitors in the clinic. While Vertex believes the forward-looking statements contained in this press release are accurate, these forward-looking statements represent the company's beliefs only as of the date of this press release and there are a number of risks and uncertainties that could cause actual events or results to differ materially from those expressed or implied by such forward-looking statements. Those risks and uncertainties include, among other things, risks related to the company’s APOL1 research and development programs, including the potential future clinical trial design and regulatory review of VX-147, that data from the company's research and development programs may not support registration or further development of its compounds due to safety, efficacy, and other risks listed under the heading “Risk Factors” in Vertex's most recent annual report and subsequent quarterly reports filed with the Securities and Exchange Commission at www.sec.gov and available through the company's website at www.vrtx.com. You should not place undue reliance on these statements, or the scientific data presented. Vertex disclaims any obligation to update the information contained in this press release as new information becomes available.
(VRTX-GEN)