LONDON--(BUSINESS WIRE)--The Flume Catheter Company Ltd. (TFCC), a medical device company dedicated to developing an improved alternative to the Foley indwelling urinary catheter, today announced that it has received clearance from the U.S. Food and Drug Administration (FDA) to market the FLUME catheter in the United States.
Approximately 30M patients in the U.S. (and 100M worldwide) continue to rely on the 1930’s Foley design for the indwelling urinary catheter, despite it being associated with infection, blockage, pain and distress for patients and significant financial burden for healthcare.
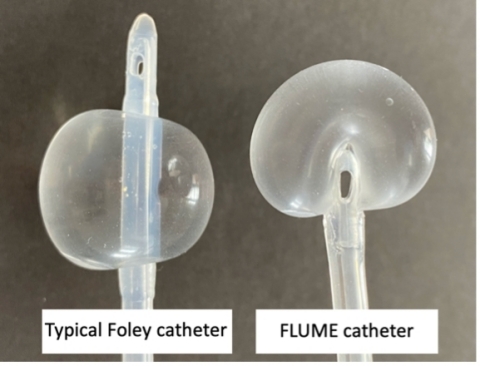
The patented FLUME catheter has the same intended use as a Foley catheter, but it is distinguished by its balloon design. Founder Dr. John Havard explains, “The balloon is designed to address some key shortcomings of the traditional Foley catheter design. When inflated, the FLUME balloon envelops the tip of the catheter so that the emptying bladder makes contact with the flexible balloon surface rather than the rigid catheter tip. The drainage ports are then deliberately inset and positioned at its base.”
A first-in-human evaluation of this alternative design is underway in the UK under Principal Investigator Prof. Marcus Drake, supported by a ‘Research for Patient Benefit’ (RfPB) award by the UK National Institute for Healthcare Research (NIHR). Results are expected towards the end of Q1 2022.
“We welcome the opportunity of evaluating this novel design with patients, in an area sorely in need of innovation,” said Prof. Drake, Professor of Physiological Urology at University of Bristol.
TFCC is looking to initiate a controlled market release in the U.S. during 2022, that will then lead to a full commercial launch.
“We are delighted to achieve 510(k) clearance and look forward to working with lead partners in the U.S. to establish the potential of FLUME catheter, which we believe will positively impact the experience of patients,” said Roger Holmes, Co-Founder & CEO.
The FLUME catheter is manufactured in the U.S. For more information; www.flumecatheter.com
About The Flume Catheter Company Ltd (TFCC)
TFCC was founded in the UK in 2016 by Suffolk family practitioner Dr. John Havard and engineer, private equity investor and former M&S CEO, Roger Holmes.
Forward-Looking Statements
This press release includes statements relating to the Flume Catheter, its FDA clearance and the Flume Catheter Company’s future plans and goals, which constitute “forward looking statements” as defined in Section 27a of the Securities Act of 1993 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. All forward looking statements are subject to risks and uncertainties that are difficult to predict and may be beyond the control of the Flume Catheter Company.