ALBUQUERQUE, N.M.--(BUSINESS WIRE)--VisionQuest Biomedical Inc. announces that it has been awarded a $2M grant from the National Institutes of Health’s National Institute of Allergy and Infectious Diseases to commercialize ASPIRE, a device that uses artificial intelligence (AI) to improve the diagnosis of cerebral malaria.
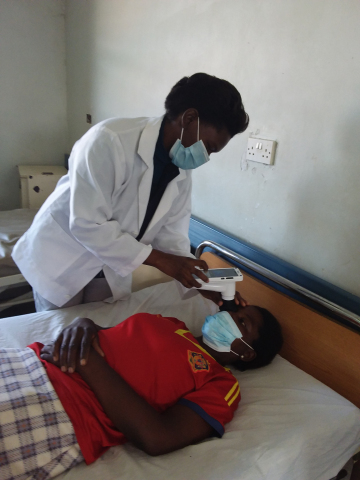
Every year, up to 100,000 children in Africa die from cerebral malaria. Up to a third of fatal cases could be prevented by a more accurate diagnosis of the disease. ASPIRE includes AI software to detect retinal biomarkers (malarial retinopathy) that are highly specific to cerebral malaria, using digital fundus images captured by a low-cost retinal camera. The software’s diagnostic performance has been reported in Nature. ASPIRE provides a user-friendly, ergonomically suitable, and affordable product, intended to be used by minimally trained health-care providers, which addresses the scarcity of ophthalmologists or specialists in the affected region.
“ASPIRE is the most technologically advanced tool to detect CM and it is going to save millions of children’s lives,” said Dr. Vinayak Joshi, Senior Scientist and Principal Investigator of the NIH grant. Dr. Simon Barriga, VisionQuest’s CEO said that “The ASPIRE software adds another tool to our product line intended to detect multiple diseases using one retinal picture.”
VisionQuest will use this NIH award to secure regulatory clearances and commercialize ASPIRE, which is currently being tested at nine malaria clinics in Malawi, Zambia, and Kenya. This award will enable VisionQuest to expand its use in clinics across four more countries: Uganda, Rwanda, Nigeria, and Ghana. These countries will be the springboard to access all malaria-affected countries in Africa.
As part of VisionQuest’s ongoing NIH awards, the company has been selected to deliver a company presentation at 2021 BIO Digital, the premier biotech event. BIO Digital is scheduled June 10-11 & 14-18, 2021.
VisionQuest expects to have a market-ready product by end of 2022 and begin sales in early 2023. This device will be made available to government hospitals, primary-care clinics, and community healthcare facilities, saving thousands of lives and reducing the effects of malaria throughout the continent. Through this project, VisionQuest is working not only to further its mission of ending preventable blindness, but also to use AI technology to improve the health and impact lives beyond vision.
About VisionQuest Biomedical Inc.: VisionQuest develops and delivers innovative artificial intelligence–based imaging technologies that increase access to health care for the people who need it the most. We serve patients and providers in the most efficient and cost-effective ways possible. Dr. Peter Soliz founded VisionQuest in 2007 to develop AI techniques that could be used by health-care professionals to evaluate digital medical photographs, specifically retinal images that showed evidence of diabetic retinopathy—the most common complication of diabetes and the leading cause of blindness in the working-age population—and other pathologies. In the United States, VisionQuest has established a network of clinics in which to study computer-based detection of retinal pathologies. In Mexico, our EyeStar software is used to screen patients for diabetic retinopathy. In the sub-Saharan country of Malawi, VisionQuest is applying retinal screening to the detection of malarial retinopathy.