SAN DIEGO--(BUSINESS WIRE)--Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a leading clinical-stage messenger RNA medicines company focused on the development of infectious disease vaccines and significant opportunities within liver and respiratory rare diseases, today announced that the Company has received approval from the Singapore Health Sciences Authority to proceed with a Phase 2 clinical study of its vaccine candidate ARCT-021. This study will build upon the favorable Phase 1/2 study results, as well as supportive preclinical data.
“We are pleased to advance ARCT-021 into a Phase 2 study based upon our promising Phase 1/2 data, which continues to support the potential for Arcturus’ STARR™ self-replicating mRNA technology to provide a highly effective, and differentiated clinical profile, including a single dose regimen,” said Steve Hughes, M.D., Chief Development Officer of Arcturus. “The Phase 2 study will enable selection of the optimal ARCT-021 vaccination regimen for Phase 3 registrational studies. We look forward to obtaining interim Phase 2 data in early 2021 providing support for the anticipated initiation of a global Phase 3 study in Q2 2021.”
“The Phase 1/2 study results, together with recently generated preclinical data, indicate that ARCT-021 leads to a potent immune response to SARS-CoV-2, and demonstrates a differentiated biological profile whereby the immune response increases in the weeks following vaccination,” said Professor Ooi Eng Eong, Emerging Infectious Diseases Programme, Duke-NUS Medical School and a member of Arcturus’ Vaccine Platform Scientific Advisory Board. “The preclinical finding that ARCT-021 results in protection from SARS-CoV-2, even when humoral immunity is depleted, is particularly notable and suggests that ARCT-021 results in highly potent cellular immunity to the virus. Together, the body of data provides us with confidence that ARCT-021 vaccination will confer substantial protection from SARS-CoV-2 infection and COVID-19.”
Summary of Phase 1/2 Interim Clinical Data
Participant Description
- 106 participants; 78 received ARCT-021; 28 received placebo
- 44 participants received ARCT-021 at doses selected for Phase 2 – 27 young adults (≤ 55 years), and 17 older adults (> 55 years)
- 34 participants (22 young adults; 12 older adults) received a single administration of 5 µg
- 10 participants (5 young adults, 5 older adults) received a single administration of 7.5 µg
- 24 participants (12 young adults, 12 older adults) received a prime-boost regimen (5 µg x 2)
Safety and Tolerability
- No safety concerns identified
- No participants have withdrawn from the study; all participants completed all doses
-
All adverse events except 2 were mild or moderate at doses selected for Phase 2
- Transient, asymptomatic Grade 3 lymphopenia seen in one participant. Lymphopenia has been observed with other RNA vaccines.
- Transient Grade 3 fatigue and myalgia observed following second injection in one older adult.
- Only serious adverse event (SAE) was in a placebo participant
Immunogenicity
-
All (44/44) subjects receiving the 5µg or7.5 µg dose had humoral immune responses for binding (Luminex) and/or neutralizing antibodies (PRNT50)
- Binding antibody titers peaked through Day 43 for 5 µg and 7.5 µg single administration groups, and Day 57 for 5 µg prime-boost groups.
- Peak geometric mean binding antibody titers: 4,959 (5 µg single shot); 6,749 (7.5 µg single shot); 16,642 (5 µg prime-boost); placebo less than 400.
- Responder peak geometric mean neutralizing antibody titers of 32, 33 and 46, following 5 µg single dose, 7.5 µg single dose and 5 µg prime-boost participants, respectively.
- Neutralizing antibody titers in convalescent sera ranged from 12 to 1818.
-
Cellular immune responses to SARS-COV-2 peptide pools evident by Day 15 for 5 µg and 7.5 µg doses
- PBMC ELISpot responses detected at Day 15.
- CD8+ and CD4+ T cell responses shown on cytokine staining (IFN-ϒ) at Day 29 following single dose at 5 µg and 7.5 µg and sustained at 2 weeks following boost at 5 µg.
- Th1 dominant CD4+ response following single dose and prime boost regimens.
New Preclinical Data Supports Protection from SARS-CoV-2
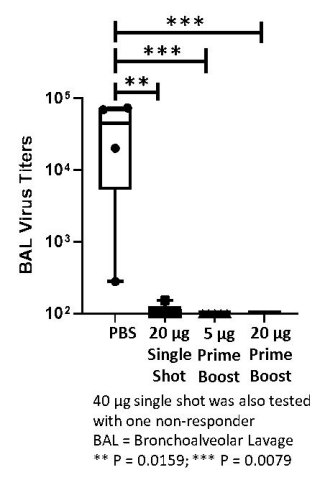
ARCT-021 vaccination effective in primate macaque challenge model
ARCT-021, both single administration and prime-boost regimens, are significantly effective in a primate challenge model. Vaccinated macaques show substantial reductions in lung viral titers. Preliminary data shows that lung viral titers are between 3.30 and 3.81 log lower in ARCT-021 vaccinated primates, in both single dose and prime-boost groups (see figure below), respectively. One week after SARS-CoV-2 virus challenge, geometric mean titers exceeded 1.31 x 104 in non-vaccinated primates compared with geometric mean titers of less than 10 in those vaccinated with ARCT-021.
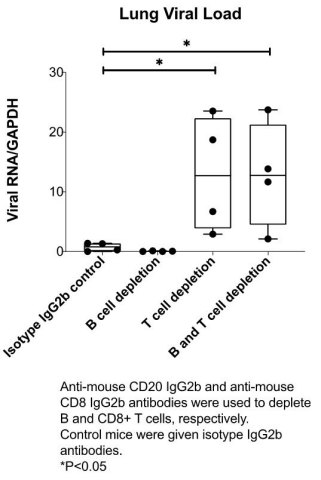
ARCT-021 vaccination effective in humoral immunodeficient mice depleted of B cells
ARCT-021 vaccination (1 ug, single administration) is protective in animals depleted of B cells (see figure below). Notably, ARCT-021 vaccination was not effective in mice depleted of CD8+ T-cells suggesting that cellular immunity, specifically CD8+ T cells, plays a critical role in preventing SARS-CoV-2 infection.
| Conference Call
Monday, Dec 28th @ 4:30 p.m. ET |
|
Domestic: |
877-407-0784 |
International: |
201-689-8560 |
Conference ID: |
13714529 |
Webcast: |
|
About Arcturus Therapeutics
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a clinical-stage mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR™ mRNA Technology and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus’ diverse pipeline of RNA therapeutic and vaccine candidates includes self-replicating mRNA vaccine programs for SARS-CoV-2 (COVID-19) and Influenza, and other programs to potentially treat Ornithine Transcarbamylase (OTC) Deficiency, Cystic Fibrosis, and Cardiovascular Disease along with partnered programs including Glycogen Storage Disease Type 3, Hepatitis B Virus, and non-alcoholic steatohepatitis (NASH). Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, replicon RNA, antisense RNA, microRNA, DNA, and gene editing therapeutics. Arcturus’ technologies are covered by its extensive patent portfolio (200 patents and patent applications, issued in the U.S., Europe, Japan, China and other countries). Arcturus’ commitment to the development of novel RNA therapeutics has led to collaborations with Janssen Pharmaceuticals, Inc., part of the Janssen Pharmaceutical Companies of Johnson & Johnson, Ultragenyx Pharmaceutical, Inc., Takeda Pharmaceutical Company Limited, CureVac AG, Synthetic Genomics Inc., Duke-NUS Medical School, and the Cystic Fibrosis Foundation. For more information visit www.ArcturusRx.com. In addition, please connect with us on Twitter and LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, including those regarding likelihood that preclinical or clinical results will be predictive of future clinical results or sufficient for regulatory approval, the likelihood of the Company to obtain clearance from regulatory authorities to proceed with planned clinical trials, the planned initiation, design or completion of clinical trials, the likelihood of success, or of the efficacy or safety, of the Company’s COVID-19 vaccine candidate or other product candidates, potential treatment regimens of the Company’s COVID-19 vaccine candidate, future operations, the likelihood of success, and the efficacy or safety, of our pipeline, including ARCT-021, the ability to initiate or complete preclinical and clinical development programs, including as a result of the COVID-19 pandemic, the ability to enroll subjects in clinical trials, the Company’s efforts to develop a vaccine against COVID-19 and therapeutic potential thereof based on the Company’s mRNA therapeutics, the continuation or success of collaborations with the Company’s strategic partners, the ability of the Company to scale up manufacturing of vaccine doses, our current cash position and expected cash burn and the impact of general business and economic conditions are forward-looking statements. Actual results and performance could differ materially from those projected in any forward-looking statements as a result of many factors including, without limitation, the impact of commercialization of third-party COVID-19 vaccines on the design, and ability to conduct, clinical trials, the availability of manufacturing capacity and raw materials, unexpected clinical results, and general market conditions that may prevent such achievements or performance. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties, including those discussed under the heading "Risk Factors" in Arcturus’ Annual Report on Form 10-K for the fiscal year ended December 31, 2019, filed with the SEC on March 16, 2020 and in subsequent filings with, or submissions to, the SEC. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.