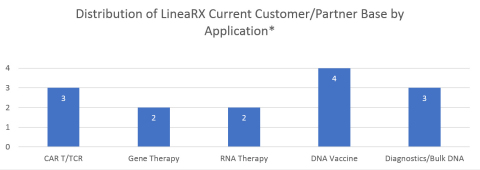
STONY BROOK, N.Y.--(BUSINESS WIRE)--Applied DNA Sciences, Inc. (NASDAQ: APDN, Applied DNA, “the Company”) announced today that LineaRx, Inc., its majority-owned subsidiary focused on next-generation biotherapeutics and diagnostics, has grown its customer base to fourteen companies currently testing its linear DNA manufacturing platform for the development of nucleic acid-based therapies and diagnostics. The Company believes this expansion is indicative of an increasing need for an alternative to plasmids as the source of DNA.
Applied DNA’s therapeutic and diagnostic client/partner base has grown to fourteen companies developing a variety of therapeutic indications including gene therapies, DNA vaccines against infectious agents and cancer, and CAR T therapies with a variety of transfection and transduction technologies, as well as several others using our platform as a template for RNA production and in vitro diagnostics “Not only is the breadth of application expanding, but our clients represent market leaders in their respective industries. Initial evaluations of our linear DNA have yielded encouraging results, and optimization is under way,” said Dr. James Hayward, CEO of Applied DNA.
“Our expansion in the commercial utility of our recent acquired (invasive Circulating Tumor Cells) iCTC-based platform for diagnostics in vitro has also attracted the attention of several companies and research institutions, and we believe these emerging opportunities will aid our growth next year,” added Dr. Hayward.
In fiscal 2020, the Company anticipates continued growth of its LineaRx client-base, while existing clients evaluate performance both in vitro and in vivo through animal studies. The Company is implementing plans to increase its manufacturing capacity and to validate manufacturing and testing procedures in compliance with FDA guidelines. The Company will also begin testing patent-pending technologies that are available for licensing that we believe may deliver higher levels of gene expression and longer functional lifetimes for the expressed proteins, as well as amplicons using episomal replication (in the nucleus, but not integrated permanently into the genome). LineaRx’s clients and partners are continuing to evolve complementary technologies, to determine the best delivery technology for linear DNA with the goal of improved therapeutic and diagnostic performance.
About LineaRx
LineaRx is commercializing the biotherapeutic value of Applied DNA’s deep expertise and experience in the design, manufacture and chemical modification of DNA by large scale polymerase chain reaction (“PCR”). Linear DNA is a form of DNA distinct from the circular form of DNA most commonly produced in plasmids and grown in bacteria. Plasmids are extrachromosomal DNA found in bacteria and are associated with the genes for antibiotic resistance which are often exchanged between bacteria and consequentially, are seen by many to embody a serious threat to global health. In addition, many nucleic acid-based therapies also rely on viral vectors for efficient transfection and expression of plasmid DNA. These viral vectors carry additional nontrivial risks and are extremely time consuming and expensive to manufacture. Go to www.adnas.com for more information on LineaRx and to learn more about how Applied DNA makes life real and safe. LineaRx is a majority-owned Applied DNA Sciences, Inc. (Nasdaq: APDN) company.
About Applied DNA Sciences
Applied DNA is a provider of molecular technologies that enable supply chain security, anti-counterfeiting and anti-theft technology, product genotyping, and pre-clinical nucleic acid-based therapeutic drug candidates.
Applied DNA makes life real and safe by providing innovative, molecular-based technology solutions and services that can help protect products, brands, entire supply chains, and intellectual property of companies, governments and consumers from theft, counterfeiting, fraud and diversion.
Visit adnas.com for more information. Follow us on Twitter and LinkedIn. Join our mailing list.
Common stock listed on NASDAQ under the symbol APDN.
Forward-Looking Statements
The statements made by Applied DNA in this presentation may be “forward-looking” in nature within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. Forward-looking statements describe Applied DNA’s future plans, projections, strategies and expectations, and are based on assumptions and involve a number of risks and uncertainties, many of which are beyond the control of Applied DNA. Actual results could differ materially from those projected due to substantial doubt relating to Applied DNA’s ability to continue as a going concern, uncertainties relating to its ability to maintain its NASDAQ listing after December 31, 2019 in light of delisting notices received and its recent hearing, the possibility of failure to make timely payment on its outstanding secured convertible notes and resulting enforcement by noteholders of remedies on collateral which includes substantially all of Applied DNA’s assets, its history of net losses, limited financial resources, limited market acceptance, its ability to penetrate key markets, the uncertainties inherent in research and development, future clinical data and analysis, including whether any of Applied DNA’s or its partners product candidates will advance further in the preclinical research or clinical trial process, including receiving clearance from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies to conduct clinical trials and whether and when, if at all, they will receive final approval from the U.S. FDA or equivalent foreign regulatory agencies, and various other factors detailed from time to time in Applied DNA’s SEC reports and filings, including our Annual Report on Form 10-K filed on December 18, 2018, as amended, our subsequent quarterly reports on Form 10-Q filed on February 7, 2019, May 9, 2019 and August 13, 2019, and other reports we file with the SEC, which are available at www.sec.gov. Applied DNA undertakes no obligation to update publicly any forward-looking statements to reflect new information, events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, unless otherwise required by law.