DANVERS, Mass.--(BUSINESS WIRE)--Abiomed (NASDAQ: ABMD) is committed to improving patient outcomes by performing FDA studies and post-market surveillance, collecting real-world evidence and identifying and sharing best practices. Impella is the most studied mechanical circulatory support device in the history of the FDA with real-world clinical data on more than 110,000 patients in the Impella Quality (IQ) Database, FDA randomized controlled trials (RCT) and post-approval studies with greater than 5,000 patients, and more than 550 peer-reviewed publications. Impella is proven to provide superior hemodynamic support over the intra-aortic balloon pump (IABP).
Today the Abiomed medical office is issuing a publication review of the observational analysis of Impella that was presented by Amin et al. at the American Heart Association Conference (AHA) on November 17. A video summarizing the review’s findings posted today at this link on ProtectedPCI.com.
The AHA analysis is flawed because:
- The data source has significant limitations, containing just a fraction of Impella patients and unable to delineate between common adverse events
- The Impella patients were much sicker and had greater baseline and procedural risks
- The analysis excluded the costliest IABP patients who were escalated to other therapies
Additionally, the conclusion is aggressive because administrative coding data cannot delineate between adverse events common to high-risk patients and device related events.
Data Source Has Significant Limitations
The AHA analysis is an observational, unaudited data analysis based on a retrospective, administrative sample coding database that does not identify device related adverse events and lumps multiple indications together, including elective high-risk and emergent cardiogenic shock cases. This makes it impossible to properly propensity match. The sample coding database only includes 4% of the Impella patients who are studied in the IQ Database.
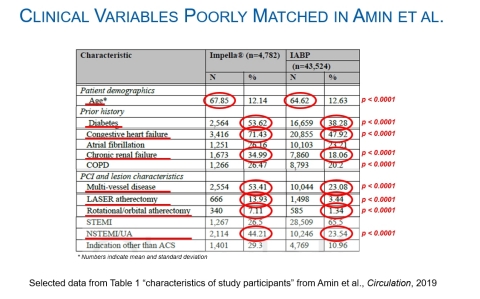
Impella Patients Were Much Sicker
Compared to the IABP patients, the Impella patients:
- Were older
- Had nearly 50% greater rate of diabetes
- Had 50% greater rate of heart failure
- Had nearly 2x the rate of chronic renal failure at baseline
- Had 2x the rate of multi-vessel disease
- Underwent 4x the rate of LASER atherectomy
- Underwent 5x the rate of rotational atherectomy
- Had nearly 2x the rate of NSTEMI
The Analysis Excluded the Costliest IABP Patients Who Were Escalated to Other Therapies
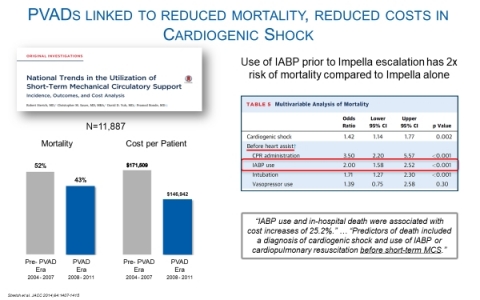
Importantly, the AHA analysis also removed patients who were escalated to other therapies, which is a major driver of costs and poor outcomes for IABP. This same flaw applies to the recent NCDR study of Impella.
A key finding from an analysis of 11,887 patients in the National Inpatient Sample (NIS) database published by Yale University colleagues Stretch et al., is that patients who were escalated from IABP had much higher cost, longer length of stay, and worse outcomes1 (see Figure 2). NIS is the largest all-payer database in the United States, containing data from Medicare, Medicaid and large commercial payers on more than seven million hospital stays.
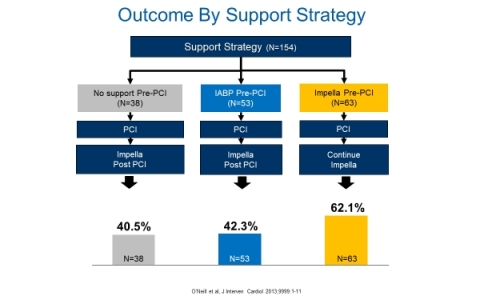
The Stretch et al. finding is consistent with the real-world Impella outcomes in cardiogenic shock published by O’Neill et al., which demonstrated a lower survival for patients who were treated with IABP prior to Impella2 (See Figure 3). Therefore, the exclusion of escalation patients from Amin et al. and the NCDR study significantly biases the IABP cohort.
The IABP-SHOCK II Randomized Controlled Trial demonstrated IABP has no hemodynamic benefit, no clinical benefit and no survival benefit. That study published in the New England Journal of Medicine and led to a Class III recommendation (not recommended, may be harmful) for IABP in cardiogenic shock in Europe and Japan.
Furthermore, the authors’ stated conclusion conflicts with the conclusions of more robust, previously published, FDA-audited, peer-reviewed, real-world studies and randomized controlled trials, which demonstrate:
- Impella is associated with improved survival in cardiogenic shock
- Impella reduces MACCE (death, stroke, MI and repeat revascularization) in high-risk PCI, when compared to IABP
- Impella enables more complete revascularization and improves ejection fraction and patient quality of life at 90 days
- Impella is associated with improved outcomes, reduced cost, reduced readmission and reduced length of stay, when compared to IABP and other therapies
An esteemed group of physicians who are leaders in the field of circulatory support have been studying Impella for more than a decade. Their research has included FDA randomized controlled trials, FDA pre- and post-market studies and physician-led initiatives such as the National Cardiogenic Shock Initiative (NCSI) Study, the INOVA Study and the Cardiogenic Shock Working Group.
William O’Neill, MD:
“The real-world, contemporary experience of the National Cardiogenic Shock Initiative prospective clinical study consistently demonstrates improvements in survival and native heart recovery with best practices that include use of Impella,” said William O’Neill, MD, the principal investigator of NCSI and the medical director of the Center for Structural Heart Disease at Henry Ford Hospital. “Payer databases, like the one used in the AHA analysis, are impossibly biased because the sicker patients will always go to Impella.”
Jeffrey Moses, MD:
“With two Impella RCTs and multiple prospective clinical post-approval studies on Impella, better data exists,” says Jeffrey Moses, MD, an interventional cardiologist, founding member of CHIP, and professor of medicine at Columbia University Medical Center who has performed more than 18,000 interventional procedures and authored more than 600 publications. “The potential for confounding in these databases presented at AHA is profound.”
Daniel Burkhoff, MD, PhD:
“With lack of granularity of patient hemodynamic and metabolic characteristics at the time of MCS use, it is impossible to compare cohorts in any meaningful way based simply on basic clinical and demographic data. Since it is well documented that Impella provides greater hemodynamic support, there is the potential for bias, with sicker patients getting Impella, as appears to be the case. Such bias cannot be overcome by statistical approaches. In addition, we know that as native heart function decreases, the amount of support by an intra-aortic balloon pump also decreases which fundamentally limits its effectiveness with failing hearts. On the contrary, Impella takes over the pumping function of the heart and unloads the left ventricle which, in several other studies, has been associated with better clinical outcomes,” says Daniel Burkhoff, MD, PhD, director, heart failure, hemodynamics and MCS research at Cardiovascular Research Foundation and primary educator of TEACH, the premier physician training and education program for learning advanced cardiovascular hemodynamics.
Alexander Truesdell, MD:
“Our data, as published in Interventional Cardiology Review and the Journal of the American College of Cardiology, suggests that multi-disciplinary care focused on prompt recognition of cardiogenic shock, rapid hemodynamic assessment, collaborative decision-making, safe vascular access, and early use of percutaneous mechanical circulatory support (primarily with the Impella family of devices), for both acute myocardial infarction and acute decompensated heart failure phenotypes, leads to significantly improved 30-day survival,” says Alexander Truesdell, MD, an interventional cardiologist at Virginia Heart and INOVA Heart and Vascular Institute.
About Impella’s FDA Approvals
Impella’s FDA Approval for High Risk PCI
The original FDA PMA approval for Impella-supported high-risk PCI in March 2015 was based on the PROTECT I FDA Trial and PROTECT II FDA Randomized Controlled Trial, as well as clinical and scientific supporting evidence from more than 215 publications, totaling 1,638 Impella patients. Further data was provided from 637 high-risk patients enrolled in the U.S. Impella registry, now called the cVAD Study. The submission also incorporated a medical device reporting analysis from approximately 14,000 Impella patients at the time. In February 2018, Impella’s FDA PMA was expanded to include patients with mild or moderately reduced ejection fraction. The data submitted to the FDA in support of the expanded PMA indication included an analysis of 230 consecutive patients with mild to moderately reduced ejection fraction from the cVAD Study.
Impella’s FDA Approval for Cardiogenic Shock
Impella’s FDA PMA for AMI cardiogenic shock was granted based on the following: Analysis of 494 cardiogenic shock patients from the U.S. Impella Registry (now called cVAD Study) and the RECOVER I FDA Study, safety and effectiveness analysis from FDA studies for high-risk PCI (PROTECT I & PROTECT II RCT), 709 high-risk PCI patients from U.S. Impella Registry, Impella literature review including 692 cardiogenic shock patients and 756 high-risk PCI patients treated with Impella, hemodynamic science demonstrating the benefits of unloading the left ventricle to reduce work and oxygen demand for the left ventricle and perfuse the end organs, the Journal of American College of Cardiology article, Hemodynamics of Mechanical Circulatory Support, Burkhoff et al., (2015), and a safety analysis of more than 25,000 Impella-treated patients using the FDA medical device reporting database, which draws from eight years (2008-2016) of U.S. experience with Impella 510(k) clearance. Furthermore, the RECOVER II RCT demonstrated the difficulty in consenting and enrolling these U.S. patients due to ethical and logistical concerns. The intra-aortic balloon pump is now a class III recommendation in Europe and Japan based on the IABP-Shock II study.
Impella’s FDA Approval for Right Heart Failure
Impella RP is the most studied right-sided device and the only percutaneous technology with FDA approval designating it as safe and effective for right heart support. Its exclusive FDA approval is a result of five years of research that included:
- RECOVER RIGHT, an FDA-approved, prospective, multicenter, single-arm study, which commenced after the company received FDA investigational device exemption (IDE) approval in November 2012 and concluded in 2014.
- HDE approval study, which was completed in January 2015
- A Continuous Access Protocol (CAP)
- FDA post-approval study, initiated after PMA approval in September 2017
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5™ with Smart Assist® are U.S. FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with real world clinical data on more than 100,000 patients and more than 550 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5™ with Smart Assist® is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com.
Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, and Impella Connect are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella 5.5, Impella ECP, CVAD Study, and SmartAssist are pending trademarks of Abiomed, Inc.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
1 Stretch, et al., Journal of the American College of Cardiology, 2014
2 O’Neill, et al., Journal of Interventional Cardiology, 2013