DANVERS, Mass.--(BUSINESS WIRE)--New research adds to 12 years of real-world data and FDA studies demonstrating the use of Abiomed's (NASDAQ: ABMD) Impella heart pumps during high-risk PCI (Protected PCI) enables skilled interventional cardiologists to achieve more complete revascularization, improved ejection fraction and quality of life, and a reduction in post-discharge adverse events.
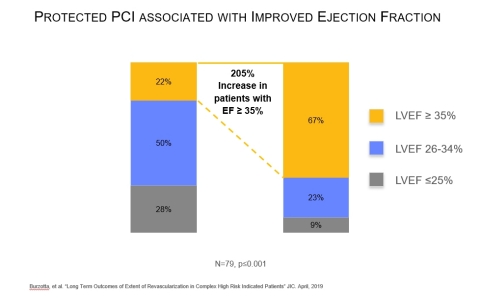
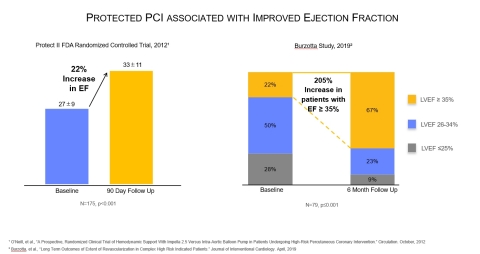
A study published on April 9 in Journal of Interventional Cardiology examined complex high-risk patients with multivessel disease who were considered unsuitable for surgery and received an Impella-supported Protected PCI. The study found six months after the Protected PCI, the number of patients with left ventricular ejection fraction (LVEF) greater than or equal to 35% increased by 205%, from 22% to 67% (n=79, p ≤ 0.001). The study also found more complete revascularization was associated with significant LVEF improvement and survival.
During a Protected PCI, the Impella heart pump is implanted minimally invasively prior to an elective or urgent high-risk percutaneous coronary intervention (PCI) to support the heart and provide hemodynamic stability. This technology enables interventional cardiologists to treat patients with severe coronary artery disease, the majority of whom have been turned down for open heart surgery.
Impella is the only hemodynamic support device that is FDA PMA approved as safe and effective for use during high-risk PCI. High-risk PCI is a first-of-its-kind FDA indication and recognizes an appropriate patient population.
The original FDA PMA approval for Impella-supported high-risk PCI in March 2015 was based on the PROTECT I FDA Trial and PROTECT II FDA Randomized Controlled Trial, as well as clinical and scientific supporting evidence from more than 215 publications, totaling 1,638 Impella patients. Further data was provided from 637 high-risk patients enrolled in the U.S. Impella registry, now called the cVAD Study. The submission also incorporated a medical device reporting analysis from approximately 14,000 Impella patients at the time. In February 2018, Impella’s FDA PMA was expanded to include patients with mild or moderately reduced ejection fraction. The data submitted to the FDA in support of the expanded PMA indication included an analysis of 230 consecutive patients with mild to moderately reduced ejection fraction from the cVAD Study.
Abiomed is an industry leader in tracking real-world evidence and identifying and validating best practices. Abiomed achieves this insight through FDA post-approval studies (PAS) embedded in the IRB-approved, prospective cVAD Study at select hospitals, and by collecting real-world data on nearly 100% of U.S. Impella patients in the Impella Quality (IQ) Database.
The IQ Database includes data on more than 36,000 Protected PCI patients who were treated from April 2009 to March 2019. New data, announced today, shows the mean and median LVEF remains below the ≤35% threshold that is considered depressed, even after Impella received its expanded FDA PMA to include patients with mild or moderately reduced ejection fraction.
| Data Source | Date Range |
Number of |
Mean |
Median |
||||
| Protect I Pilot Trial and Protect II FDA Randomized Control Trial | 2006 - 2012 | 245 | 25% | 23% | ||||
|
IQ Database:
Original FDA PMA Indication - Depressed EF (≤35%) |
April 1, 2009 – February 14, 2018 | 25,214 | 25% | 25% | ||||
|
IQ Database:
After Expanded FDA PMA Indication for Mild and Moderately Depressed EF with Complex Anatomy and Comorbidities (up to ~50% EF) |
February 15, 2018 – March 30, 2019 | 11,096 | 30% | 30% |
Note: On February 14, 2018, Impella’s FDA PMA indication was expanded to include patients with mild and moderately depressed EF with complex anatomy and comorbidities2.
Impella is the only FDA approved therapy for the estimated 121,000 U.S. high-risk PCI patients3 who qualify each year for the FDA’s definition of a high-risk PCI. Currently, Abiomed treats approximately 10% of the eligible population. Furthermore, a 2016 study by Doshi, et al. that published in the Journal of the American College of Cardiology found, “it can be estimated that every year more than 325,000 patients with new-onset HF (heart failure) and CAD (coronary artery disease) might not be adequately assessed for ischemic CAD4.”
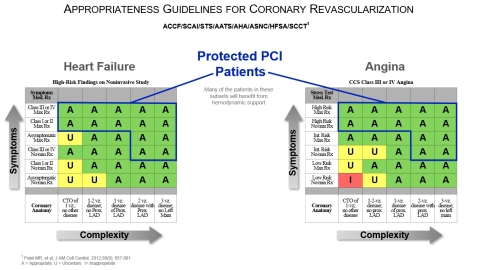
The charts below show patient populations which include patients who are appropriate for Protected PCI.
Impella enables experienced interventional cardiologists to achieve complete revascularization and improved patient quality of life as demonstrated in multiple studies and publications:
- Journal of Cardiovascular Medicine, 2008 - Impella patients studied by Burzotta et al., had an improved LVEF at one year (from 31 ± 7% to 41% ± 13%, p=0.02).
- Journal of the American College of Cardiology, 2009 - The PROTECT I trial found patients who had a Protected PCI with Impella had a 31% improvement in LVEF at 30 day follow up. (from 26 ± 6% to 34 ± 11%, p = 0.003).
- Circulation, 2012 - The PROTECT II Randomized Controlled Trial found Protected PCI with Impella led to a 58% improvement in NYHA class III and IV heart failure symptoms at 90 days (p<0.001). The trial also found, during follow-up after Protected PCI with Impella, patients had a 22% improvement in LVEF (p<0.001).
- Catheterization Cardiovascular Interventions, 2012 – Maini, et al., found a 17% improvement in LVEF at follow up, after a Protected PCI with Impella (p<0.0001).
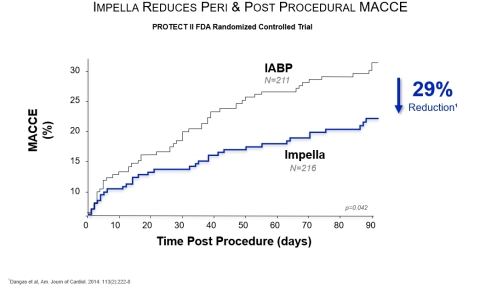
- American Journal of Cardiology, 2014 - An analysis of the PROTECT II Randomized Controlled Trial by Dangas, et al., found Impella use led to a 29% reduction in major adverse cardiac and cerebrovascular events (MACCE) at 90 days, compared to the use of the intra-aortic balloon pump (IABP) (p=0.042).
- Journal of Interventional Cardiology, 2015 - Kovacic, et al., found Impella maintains patient hemodynamics, allowing for more complete revascularization and improved outcomes. For example, when three vessels were treated, the procedural decrease in arterial pressure from baseline was -18.8% for IABP, compared to -7.6% for Impella (p=0.026).
- The American Journal of Cardiology, 2018 – Alaswad, et al., compared patients with LVEF > 35% to patients with LVEF ≤ 35% and found, despite high-risk features, MACCE rates were favorable, with no differences between the two groups (3.48% vs 4.54%; p=0.574).
- Journal of Interventional Cardiology, 2019 – Burzotta, et al., found Protected PCI with Impella is associated with LVEF improvement in complex high-risk patients. The authors also found more complete revascularization is associated with increased LVEF and survival.
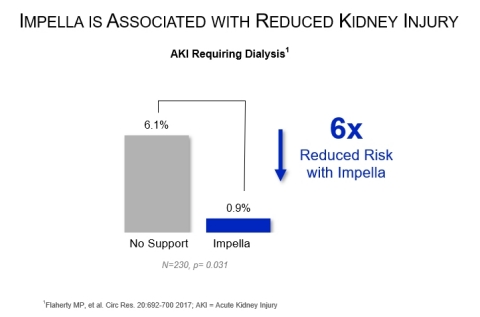
Acute kidney injury (AKI) is lower in Impella patients undergoing high-risk PCI:
- Circulation, 2012 - The PROTECT II Randomized Controlled Trial found, despite higher levels of contrast in the Impella arm, acute renal dysfunction was lower in the Impella arm at 30 days and 90 days (p=0.792 and p=0.776, respectively).
- Circulation Research, 2017 - Flaherty, et al., identified a six-fold reduction in AKI requiring dialysis when Impella support was used, compared to without Impella support (p=0.031).
- Journal of the American College of Cardiology, 2019 – Flaherty et al., found, compared to a predicted rate of 22%, only 7% of Impella-supported patients developed AKI at 72 hours, representing a 70% lower AKI risk (p<0.0001).
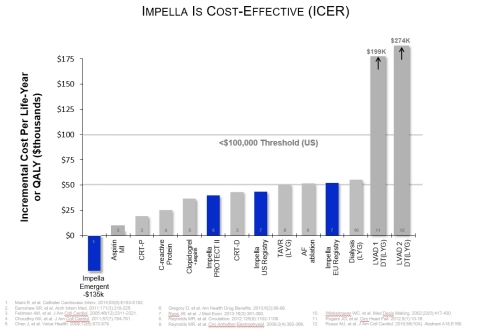
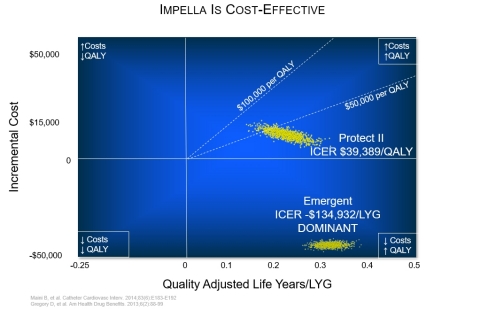
Impella is cost effective:
- American Drug & Health Benefits, 2013 - Gregory, et al., found Impella had a 52% reduction in readmissions from repeat revascularization (p=0.024) and a 22% reduction in length of stay (p=0.008), compared to IABP use.
- Journal of the American College of Cardiology, 2014 – Stretch, et al., found in cases of cardiogenic shock in coronary atherosclerosis and other heart disease, percutaneous ventricular assist devices (pVADs) reduced costs by $54,000 per case (p=0.015) and reduced mortality by 58%.
- Expert Review of Pharmacoeconomics & Outcomes Research, 2014 – An evaluation of six studies of the economic impact and relative value of pVADs finds they are cost effective in the long-term.
Patients who have benefited from Protected PCI with Impella include Mongin Smyly, whose constant fatigue and weakness left him feeling hopeless. That’s when he was identified as an appropriate candidate for a Protected PCI with Impella. Following his procedure, Mr. Smyly experienced an improved quality of life and was able to return to work. He said, “Following the Protected PCI procedure, I felt better than I had in a long time. Today, I am back to enjoying life with my wife and family and I am beyond grateful.”
"The scientific evidence clearly demonstrates the advantages of conducting a Protected PCI in patients who have compromised hemodynamics, complex coronary artery disease and comorbidities. In the past, these patients would have had minimal to no other treatment options,” said Ayaz Rahman, MD, the interventional cardiologist at Parkwest Medical Center who treated Mr. Smyly. “Protected PCI with Impella allows for more complete revascularization and better long-term outcomes. It also improves the patient’s quality of life and reduces readmissions."
ABOUT IMPELLA HEART PUMPS
The Impella 2.5 and Impella CP devices are FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5®, Impella CP®, Impella CP® with SmartAssist, Impella 5.0® and Impella LD® are FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit: www.protectedpci.com.
Impella is the most studied mechanical circulatory support device in the history of the FDA and has exclusive PMA approvals for high-risk PCI, as a therapy to allow for native heart recovery after cardiogenic shock derived from AMI or cardiomyopathy, and right ventricular heart failure.
The ABIOMED logo, ABIOMED, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, Impella Connect, and Recovering hearts. Saving lives. are registered trademarks of ABIOMED, Inc. in the U.S. and in certain foreign countries.
ABOUT ABIOMED
Based in Danvers, Massachusetts, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the Company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The Company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the Company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The Company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
1Patients with ejection fraction values
2 On
February 14, 2018, Impella’s FDA PMA indication was expanded to read:
The Impella 2.5®, Impella CP® and Impella CP®
with SmartAssist® Systems are temporary (≤ 6 hours)
ventricular support devices indicated for use during high-risk
percutaneous coronary interventions (PCI) performed in elective or
urgent, hemodynamically stable patients with severe coronary artery
disease, when a heart team, including a cardiac surgeon, has determined
high-risk PCI is the appropriate therapeutic option. Use of the Impella
2.5, Impella CP, and Impella CP with SmartAssist Systems in these
patients may prevent hemodynamic instability, which can result from
repeat episodes of reversible myocardial ischemia that occur during
planned temporary coronary occlusions and may reduce peri- and
post-procedural adverse events.
3 American Heart
Association, Heart Disease & Stroke Statistics, 2015 Update; Gheorghiade
et al. Circulation; Braunwald, et al. JACC; Dehmer, et al. JACC;
Peterson NEJM 2010; Farmer et al. JACC Cardiovasc Imaging.
2014;7(7):690-700.
4 Doshi D, et al. J Am Coll Cardiol.
2016;68(5):450-458.