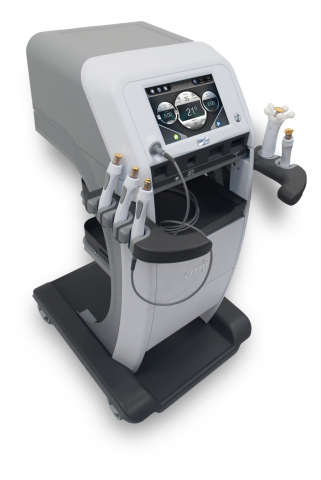
MARLBOROUGH, Mass.--(BUSINESS WIRE)--Hologic, Inc.’s (Nasdaq: HOLX) Cynosure division announced today the North American launch of the FDA-cleared TempSure™ Surgical RF technology, a new offering of the TempSure™ radiofrequency (RF) platform that provides clinicians the ability to perform both surgical and non-surgical aesthetic procedures across a variety of specialties, on a single device.
Cynosure also has returned TempSure™ Vitalia hand pieces and probes to the market and will continue to market its MonaLisa Touch® CO2 laser following the U.S. Food and Drug Administration (FDA) inquiry on products used in energy-based women’s health procedures. Over the past several months, Cynosure worked closely with the FDA and reviewed and updated all its marketing and promotional materials to ensure they are consistent with the FDA’s labeling expectations.
TempSure Surgical RF technology harnesses a 300-watt and 4-MHz radiofrequency platform that enables precise incisions with minimal lateral thermal damage to surrounding tissues. The resulting high-quality coagulation lessens sparking and charring during procedures, which promotes quicker recovery and better healing for patients1. The device is designed to improve patient satisfaction and aesthetic outcomes, and can be used by clinicians across a variety of specialties including plastic surgery, dermatology, gynecology and ophthalmology.
“We’re continually innovating to ensure our customers set themselves apart with effective and diverse treatment offerings, and this enhanced platform is a gamechanger across specialties,” said Kevin Thornal, Hologic's Division President, Cynosure. “The cutting-edge technology of the TempSure platform will now allow doctors to transition seamlessly from invasive to non-invasive treatments on one device.”
Thornal added, “We’re pleased to reintroduce TempSure Vitalia and to continue offering MonaLisa Touch to provide clinically strong products for women’s wellness. Cynosure is the only medical aesthetic manufacturer to offer two different energy-based modalities for women’s wellness — radiofrequency and CO2 — and we remain committed to advancing women’s pelvic health around the globe.”
TempSure Surgical RF technology is designed to enhance the existing TempSure radiofrequency platform, and includes a variety of electrodes that integrate seamlessly with the main TempSure unit. In January 2018, Cynosure launched the TempSure radiofrequency platform with TempSure™ Envi, a device for treating facial fine lines and wrinkles, tightening the skin through soft tissue coagulation, and temporarily reducing the appearance of cellulite. New and existing customers now have the opportunity to customize their TempSure Envi system to include TempSure Surgical RF technology, TempSure Vitalia hand pieces, or a combination of all three to help meet different clinical needs at their practice.
“TempSure Surgical is truly a breakthrough technology that has improved my surgical procedures and delivered fantastic results,” said Dr. Barry DiBernardo of New Jersey Plastic Surgery. “I immediately noticed cleaner cuts and optimum coagulation during surgery, which gives me the confidence to tell my patients they will experience less bruising and better wound healing. With the addition of TempSure Surgical, I am now able to perform improved surgical incisions and also offer noninvasive wrinkle reduction with TempSure Envi. This technology has revolutionized my practice — and it will revolutionize your practice.”
To learn more about the TempSure radiofrequency platform, please visit www.cynosure.com/tempsure-platform.
About Hologic, Inc.
Hologic, Inc. is an innovative medical technology company primarily focused on improving women’s health and well-being through early detection and treatment. For more information on Hologic, visit www.hologic.com.
Hologic and The Science of Sure, and associated logos are registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries. Cynosure is a registered trademark of Cynosure, Inc. and TempSure is a trademark of Cynosure, Inc. MonaLisa Touch is a registered trademark owned by DEKA.
Forward-Looking Statements
This news release may contain forward-looking information that involves risks and uncertainties, including statements about the use of Hologic products. There can be no assurance these products will achieve the benefits described herein or that such benefits will be replicated in any particular manner with respect to an individual patient, as the actual effect of the use of the products can only be determined on a case-by-case basis. In addition, there can be no assurance that these products will be commercially successful or achieve any expected level of sales. Hologic expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any such statements presented herein to reflect any change in expectations or any change in events, conditions or circumstances on which any such data or statements are based.
1 With less tissue destruction, healing is accelerated and patients can recover quickly. Bridenstine, J.B., Derm Surgery (1998); vol 24, p397-400.
SOURCE: Hologic, Inc.