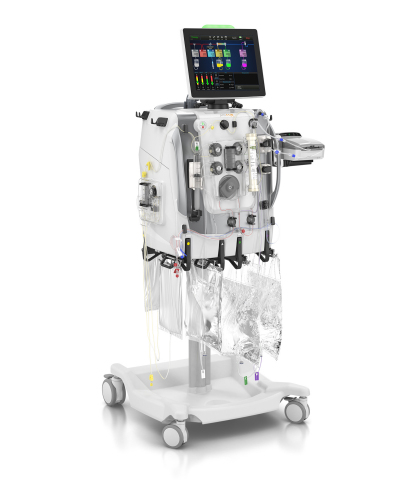
DEERFIELD, Ill.--(BUSINESS WIRE)--Baxter International Inc. (NYSE:BAX), a global leader in acute care, announced today the company received CE mark of both its PrisMax system and its TherMax blood warmer. PrisMax is the company’s next-generation technology for continuous renal replacement and organ support therapies. Designed with input from more than 650 healthcare practitioners around the world, the PrisMax system features innovative technology to make delivering therapy simpler and more efficient while also improving treatment accuracy in the ICU. Baxter kicked off the launch of the PrisMax system at the 2018 European Society of Intensive Care Medicine (ESICM) congress in Paris last week.
“Baxter has been a leader in continuous renal replacement therapy (CRRT) technology for more than 20 years. We’ve built on this expertise to bring to market the most advanced technology currently available that directly addresses what clinicians have said they need to better treat patients: simplicity, efficiency and accuracy,” said Reaz Rasul, general manager of Baxter’s Acute Therapies business. “The PrisMax system is foundational to accomplishing our shared vision to help transform care for critically ill patients and establish best-in-class treatments for a variety of organ support therapies.”
Building on Baxter’s leading Prismaflex technology currently used by hospitals in more than 90 countries, the PrisMax system, in combination with specialty dialyzers, delivers a complete range of extracorporeal (outside the body) therapies to remove waste products, excess fluids and inflammatory mediators, to help manage patients with acute kidney injury (AKI), and as an aid in sepsis management. AKI is an increasingly common complication of acute illnesses in intensive care units and hospitals.1 2 3
“Our assessment of the PrisMax system across seven ICUs in six countries concluded that the device features several important enhancements that contribute to safety, efficiency and user friendliness. The launch of PrisMax is a big step forward for clinicians’ abilities to treat patients in the ICU more effectively,” said Marcus Broman, M.D., Department of Perioperative and Intensive Care of Skåne University Hospital in Lund, Sweden, and lead author of a publication about the results.4
The prospective, multicenter, international pilot study found that PrisMax delivered significant improvements in areas that impact efficiency and ease of use, including the time needed for bag changes, the number of informational and malfunction alarms, how often the blood pump stops, filter life and machine downtime. 4 The findings were published earlier this year in Blood Purification. 4
The TherMax blood warmer, which is used alongside the PrisMax system, is a critical component for extracorporeal therapies as a patient’s blood is purified outside of the body and must be at a certain temperature prior to returning to the body. TherMax uses a bi-directional connection with the PrisMax system to help meet warming targets by automatically adjusting heating to meet the prescribed return blood temperature. 5 The TherMax blood warmer also includes several advanced patient safety features to help control blood return temperature, detect leaks, and to ensure the correct setup.5
Baxter intends to commercially launch PrisMax and TherMax in more than 19 countries across Europe, with hospitals in Denmark, France, Italy and Sweden to be among the first to receive the new devices.
About Our Acute Therapies Portfolio
A leader in multi-organ support therapy options, Baxter has been at the forefront of advancing new technologies and revolutionizing treatment for critically ill patients around the world. Baxter’s leading Prismaflex system has offered clinicians the flexibility to meet patients’ diverse needs and powered our portfolio of products to deliver a complete range of extracorporeal (outside the body) blood purification therapies to help manage patients with acute kidney injury (AKI),and with our specialty dialyzers aid in the management of sepsis. PrisMax, Baxter’s next generation system, builds on our legacy of proven performance with enhancements designed to improve accuracy and system performance while making it simpler and more efficient for clinicians to deliver therapy.
About Baxter
Every day, millions of patients and caregivers rely on Baxter’s leading portfolio of critical care, nutrition, renal, hospital and surgical products. For more than 85 years, we’ve been operating at the critical intersection where innovations that save and sustain lives meet the healthcare providers that make it happen. With products, technologies and therapies available in more than 100 countries, Baxter’s employees worldwide are now building upon the company’s rich heritage of medical breakthroughs to advance the next generation of transformative healthcare innovations. To learn more, visit www.baxter.com and follow us on Twitter, LinkedIn and Facebook.
Rx Only. For safe and proper use of this device, refer to the full Instructions for Use.
This release includes forward-looking statements concerning PrisMax and TherMax, including potential benefits associated with their use and information about planned commercial launches. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: satisfaction of regulatory and other requirements; actions of regulatory bodies and other governmental authorities; product quality, manufacturing or supply, or patient safety issues; changes in law and regulations; and other risks identified in Baxter's most recent filing on Form 10-K and other SEC filings, all of which are available on Baxter's website. Baxter does not undertake to update its forward-looking statements.
Baxter, PrisMax, TherMax and Prismaflex are registered trademarks of Baxter International Inc.
1 Siew ED, Davenport A: The growth of acute kidney injury: a
rising tide or just closer attention to detail? Kidney Int 2015;87:46-61.
2
O'Connor ME, Kirwan CJ, Pearse RM, Prowle JR: Incidence and associations
of acute kidney injury after major abdominal surgery. Intensive Care Med
2016;42:521-530.
3 Susantitaphong P, Cruz DN, Cerda J,
Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL: World incidence of
AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482-1493.
4
Broman M, et al. Blood Purif. 2018;46:220-227.
5
Baxter - TherMax Operators Manual. AW7006 Rev A 2018;Sep.