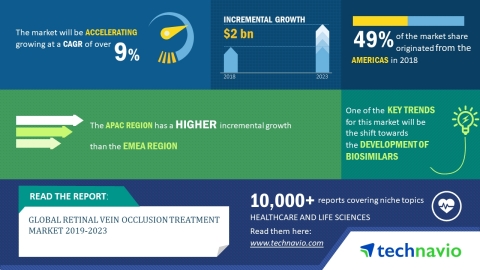
LONDON--(BUSINESS WIRE)--The global retinal vein occlusion treatment market 2019-2023 is expected to post a CAGR of more than 9% during the forecast period, according to the latest market research report by Technavio.
A key factor driving the growth of the market is strong pipeline and recent drug approvals. The drug development process is a long and expensive process. Before entering the market, therapeutics undergo various clinical trials and then get approved by regulatory authorities such as the United States Food and Drug Association (US FDA) and the European Medicines Agency (EMA). Most of the therapeutics that are in last stage of development are expected to get marketing approval during the forecast period.
This market research report on the global retinal vein occlusion treatment market 2019-2023 also provides an analysis of the most important trends expected to impact the market outlook during the forecast period. Technavio classifies an emerging trend as a major factor that has the potential to significantly impact the market and contribute to its growth or decline.
This report is available at a USD 1,000 discount for a limited time only: View market snapshot before purchasing
In this report, Technavio highlights the shift toward the development of biosimilars as one of the key emerging trends in the global retinal vein occlusion treatment market:
Global retinal vein occlusion treatment market: Shift toward the development of biosimilars
Biosimilars are generic versions of biologics and are also known as reference products. Biosimilars are approved by the US FDA. The main advantage of biosimilars is that they are about 20% less expensive than biologics. Fewer clinical trials are needed to be performed for biosimilars compared with biologics, which is the main reason for their lesser cost.
“The patent of some of the branded RVO drugs is expected to expire soon, which will increase the demand for biosimilars. For instance, Formycon is developing both Lucentis and EYLEA biosimilars. FYB201 is a biosimilar of Lucentile, which is currently in the Phase III stage of development. the shift toward the development of biosimilars is expected to fuel the growth of the global RVO treatment market during the forecast period,” says a senior analyst at Technavio for research on infectious and rare diseases.
Global retinal vein occlusion treatment market: Segmentation analysis
This market research report segments the global retinal vein occlusion treatment market by end-user (BRVO and CRVO) and geographical regions (APAC, EMEA, and the Americas).
The Americas led the market in 2018 with a market share of more than 49%, followed by APAC and EMEA respectively. The region is expected to continue to dominate the market during the forecast period.
Looking for more information on this market? Request a free sample report
Technavio’s sample reports are free of charge and contain multiple sections of the report such as the market size and forecast, drivers, challenges, trends, and more.
Some of the key topics covered in the report include:
Market Landscape
- Market ecosystem
- Market characteristics
- Market segmentation analysis
Market Sizing
- Market definition
- Market size and forecast
Five Forces Analysis
Market Segmentation
Geographical Segmentation
- Regional comparison
- Key leading countries
Market Drivers
Market Challenges
Market Trends
Vendor Landscape
- Vendors covered
- Vendor classification
- Market positioning of vendors
- Competitive scenario
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 10,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
If you are interested in more information, please contact our media team at media@technavio.com.