STRASBOURG, France--(BUSINESS WIRE)--Regulatory News:
Transgene (Paris:TNG) (Euronext Paris: TNG) a biotechnology company that designs and develops virus-based immunotherapies against cancers and infectious diseases, announces myvacTM, an individualized, viral vector-based immunotherapy against cancer that will enter clinical development in 2019.
myvacTM, an individualized, MVA-based
immunotherapy
myvacTM is designed to
stimulate and educate the immune system of patients to recognize and
destroy tumor cells. The personalized immunotherapy product is based on
the mutations that are identified in the patient’s own tumor. These
mutations are highly relevant targets since they lead to the expression
of tumor neoantigens which are known to trigger a stronger immune
response than “classic”2 tumor antigens.
Once administered to the patient, myvacTM triggers a cascade of immune responses against a variety of targets found in the cancer cells.
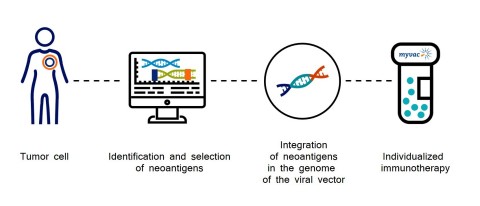
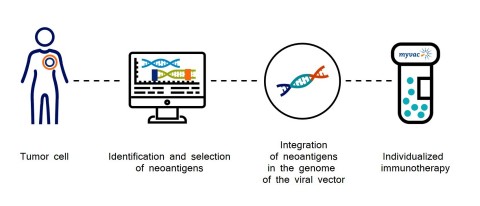
The neoantigens which are the basis for the myvacTM approach are identified by sequencing and selected using artificial intelligence algorithms, and then integrated into the genome of the viral vector (MVA).
myvacTM differs from autologous treatments since no biological material from the patient is used in the manufacturing process and as such is much easier to manufacture; it is a truly individualized approach that uses the information that is specific to the characteristics of each patient’s tumor.
Transgene has combined its expertise in viral vectors with highly innovative technologies to develop myvacTM.
myvacTM features several key advantages:
- It is expected to deliver the benefits of an individualized treatment without the disadvantages of autologous approaches (Transgene does not modify the patient’s cells but integrates the neoantigen panel into the virus);
- It is based on a viral strain (MVA) whose safety, tolerability, immunogenicity and efficacy have already been demonstrated in the clinic with TG4010 and TG4001;
- The myvacTM viral vector (MVA) has repeatedly shown that it can induce a strong immune response from the patient against the tumor antigens incorporated in its viral genome as well as an enlargement of the antitumoral immune repertoire (epitope spreading);
- An “all-in-one” formula, requiring neither adjuvant nor association of different peptides.
Éric Quéméneur, PhD, Executive VP, Chief Scientific Officer of Transgene, said: “With myvacTM, Transgene is at the forefront of innovation in cancer immunotherapy. Based on our know-how in virotherapy, we have successfully integrated sequences coding for neoantigens to create an individualized immunotherapy. By combining sequencing and artificial intelligence with the design of the virus, myvacTM marks the entry of viral vector-based approaches in the era of digital transformation. Importantly we have also set up an organization able to design and manufacture myvacTM for each patient in a timely and cost-competitive manner. The myvacTM innovation is a logical evolution of our expertise and a new therapeutic option that promises a major improvement over existing therapies. myvacTM is also the result of our policy of open innovation which is based on working with partners developing technologies that are complementary to our expertise allowing us to benefit from a multidisciplinary approach. We look forward to demonstrating the transformative potential of myvacTM in the clinical trials we plan to start in 2019.”
myvacTM, an ambitious project
expected to enter the clinic in 2019
myvacTM
will be administered to patients with solid tumors. Two clinical trials
are being set up in Europe and in the United States, including
HPV-negative head and neck cancers and ovarian cancer. These trials are
expected to start in 2019.
The first preclinical and translational
results will be presented soon at immuno-oncology conferences.
Our innovative network combines bioengineering and digital transformation
To design myvacTM, Transgene and its collaborative network had to overcome many scientific and technical challenges. The network’s expertise covers all the required know-how:
- Institut Curie (Cancer Immunotherapy Center, led by Professor Amigorena) is involved in the generation of translational data and the characterization of the mechanism of action;
- HalioDx studies biomarkers to maximize the effectiveness of the therapy;
- Traaser automates, secures and manages genomic data, including predictive algorithms provided by a recognized partner in artificial intelligence;
- Transgene has developed a unique manufacturing pilot unit in France to vectorize neoantigens and provide myvacTM within a timeframe compatible with clinical treatment schemes.
This innovative project has obtained the labeling of the Biovalley France and Eurobiomed French competitiveness clusters.
Transgene holds the intellectual property of the myvacTM viral vector platform and is actively working on the translational development of this innovative technology.
About Transgene
Transgene (Euronext: TNG), part of
Institut Mérieux, is a publicly traded French biotechnology company
focused on designing and developing targeted immunotherapies for the
treatment of cancer and infectious diseases. Transgene’s programs
utilize viral vector technology with the goal of indirectly or directly
killing infected or cancerous cells. The Company’s lead clinical-stage
programs are: TG4010, a therapeutic vaccine against non-small cell lung
cancer, Pexa-Vec, an oncolytic virus against liver cancer, and TG4001, a
therapeutic vaccine against HPV-positive head and neck cancers. The
Company has several other programs in clinical development, including
TG1050 (a therapeutic vaccine for the treatment of chronic hepatitis B)
and TG6002 (an oncolytic virus for the treatment of solid tumors).
With
its proprietary Invir.IOTM, Transgene builds on its expertise
in viral vectors engineering to design a new generation of
multifunctional oncolytic viruses.
MyvacTM, an
individualized MVA-based immunotherapy integrating neoantigens,
completes this innovative research portfolio.
Additional
information about Transgene is available at www.transgene.fr.
Follow
us on Twitter: @TransgeneSA
Disclaimer
This press release contains
forward-looking statements, which are subject to numerous risks and
uncertainties, which could cause actual results to differ materially
from those anticipated. The occurrence of any of these risks could have
a significant negative outcome for the Company’s activities,
perspectives, financial situation, results, regulatory authorities’
agreement with development phases, and development. The Company’s
ability to commercialize its products depends on but is not limited to
the following factors: positive pre-clinical data may not be predictive
of human clinical results, the success of clinical studies, the ability
to obtain financing and/or partnerships for product manufacturing,
development and commercialization, and marketing approval by government
regulatory authorities. For a discussion of risks and uncertainties
which could cause the Company’s actual results, financial condition,
performance, or achievements to differ from those contained in the
forward-looking statements, please refer to the Risk Factors (“Facteurs
de Risque”) section of the Document de Référence, available on the AMF
website (http://www.amf-france.org)
or on Transgene’s website (www.transgene.fr).
Forward-looking statements speak only as of the date on which they are
made and Transgene undertakes no obligation to update these
forward-looking statements, even if new information becomes available in
the future.
1 Tumor neoantigens: genetic mutations of tumor cells that
are specific to the patient. Unlike “classic” tumor antigens that sign
the identity of a type of tumor, neoantigens are unique.
2
Chen DS, Mellman I., Elements of cancer immunity and the
cancer-immune set point, Nature (2017) 541:321–30.10.1038/nature21349