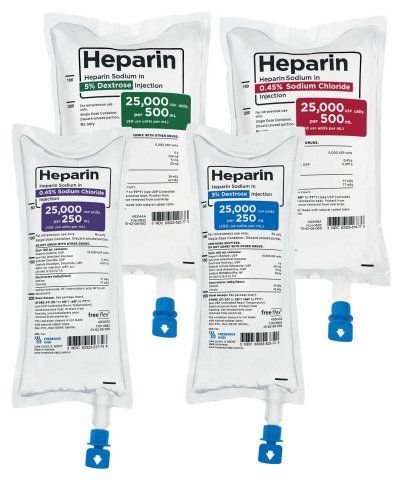
LAKE ZURICH, Ill.--(BUSINESS WIRE)--Fresenius Kabi announced today it has introduced four new presentations of Heparin Sodium in the company’s proprietary ready-to-administer freeflex® containers. Fresenius Kabi introduced the advanced IV-container technology in the United States in 2013, and has been expanding its portfolio of convenient, ready-to-administer medicines in non-DEHP bags as well as in prefilled syringes.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition.
“Clinicians continue to look for convenient, ready-to-administer products to help minimize drug errors and assure patient safety,” said John Ducker, president and CEO of Fresenius Kabi USA. “We remain focused on meeting these needs by expanding the number of products we offer in both freeflex bags and prefilled syringes. With this most recent offering we’re also proud to offer the most comprehensive portfolio of Heparin Sodium in the U.S.”
Fresenius Kabi offers Heparin Sodium in 11 vial presentations and one pre-filled syringe presentation, in addition to the new freeflex offerings, which include:
- Heparin Sodium in 0.45% NaCl freeflex 250 mL; 25,000 USP units per 250 mL
- Heparin Sodium in 0.45% NaCl freeflex 500 mL; 25,000 USP units per 500 mL
- Heparin Sodium in 5% Dextrose freeflex 250 mL; 25,000 USP units per 250 mL
- Heparin Sodium in 5% Dextrose freeflex 500 mL; 25,000 USP units per 500 mL
Freeflex is a non-PVC, non-DEHP multilayer polyolefin film flexible bag for infusion solutions with patented port technology, clear labeling, a self-sealing septum and an oversized break-off cap that indicates the directional flow of solutions.
INDICATIONS AND USAGE
Heparin Sodium is an anticoagulant indicated for:
- Prophylaxis and treatment of venous thromboembolism and pulmonary embolism
- Atrial fibrillation with embolization
- Treatment of acute and chronic consumptive coagulopathies (disseminated intravascular coagulation)
- Prevention of clotting in arterial and cardiac surgery
- Prophylaxis and treatment of peripheral arterial embolism
- Anticoagulant use in blood transfusions, extracorporeal circulation, and dialysis procedures.
IMPORTANT SAFETY INFORMATION
Heparin Sodium is contraindicated in patients with history of Heparin-Induced Thrombocytopenia (HIT) and Heparin-Induced Thrombocytopenia and Thrombosis (HITT), known hypersensitivity to heparin or pork products, those whom suitable blood coagulation tests cannot be performed at appropriate intervals, and patients with uncontrolled bleeding states, except when this is due to disseminated intravascular coagulation.
Fatal Medication Errors: Heparin is supplied in various strengths. Fatal hemorrhages have occurred due to medication errors. Confirm choice of correct strength prior to administration.
Hemorrhage: Fatal cases have occurred. Avoid using heparin in the presence of major bleeding, except when the benefits of heparin therapy outweigh the potential risks.
Heparin-Induced Thrombocytopenia (HIT) and Heparin-Induced Thrombocytopenia and Thrombosis (HITT): Monitor for signs and symptoms and discontinue if indicative of HIT and HITT.
Thrombocytopenia: Can occur 2 to 20 days (average 5 to 9) following the onset of heparin therapy. Monitor thrombocytopenia of any degree closely. If the count falls below 100,000/mm3 or if recurrent thrombosis develops, promptly discontinue heparin, evaluate for HIT and, if necessary, administer an alternative anticoagulant.
Coagulation Testing and Monitoring: When using a full dose heparin regimen, adjust the heparin dose based on frequent blood coagulation tests. Periodic platelet counts, hematocrits are recommended during the entire course of heparin therapy.
Heparin Resistance: Increased resistance to heparin is frequently encountered in fever, thrombosis, thrombophlebitis, infections with thrombosing tendencies, myocardial infarction, cancer, in postsurgical patients, and patients with antithrombin III deficiency. Close monitoring of coagulation tests is recommended in these cases. Adjustment of heparin doses based on anti-Factor Xa levels may be warranted.
Hypersensitivity Reactions: Heparin sodium is derived from animal tissue, monitor for signs and symptoms of hypersensitivity when it is used in patients with a history of allergy. Heparin Sodium in 5% Dextrose Injection contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people.
Most common adverse reactions are hemorrhage, thrombocytopenia, HIT and HITT, hypersensitivity reactions, and elevations of aminotransferase levels.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions: Drugs that interfere with coagulation, platelet aggregation or drugs that counteract coagulation may induce bleeding.
Overdosage: Bleeding may result from heparin overdosage and may require a neutralization of heparin effect.
This Important Safety Information does not include all the information needed to use Heparin Sodium in 0.45% Sodium Chloride Injection and Heparin Sodium in 5% Dextrose Injection safely and effectively. Please click on the following link https://tinyurl.com/y72s9xv6 for the full prescribing information for Heparin Sodium in 0.45% Injection and Heparin Sodium in 5% Dextrose Injection or at www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. For more information about Fresenius Kabi worldwide, please visit www.fresenius-kabi.com.