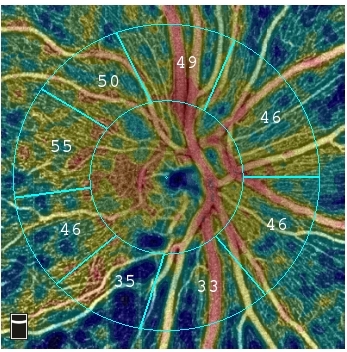
FREMONT, Calif.--(BUSINESS WIRE)--Optovue announced FDA 510(k) clearance of AngioAnalytics™, the world’s first optical coherence tomography angiography (OCTA) blood vessel measurement technology to help clinicians manage diseases that cause progressive blindness. The company also received clearance for its three-dimensional projection artifact removal (3D PAR) software, which greatly improves OCTA image quality and enables accurate measurement and interpretation of OCTA images. AngioAnalytics and 3D PAR are commercially available in the U.S.
“AngioAnalytics and 3D PAR are game changers because they 'erase' overlying inner retinal blood vessel artifacts allowing for better visualization and more accurate measurement of the outer retina and choroidal structures, essential in treating the abnormal blood vessel growth (CNV membrane) that leads to blindness for patients with wet age-related macular degeneration (AMD),” said Paul E. Tornambe, M.D., F.A.C.S., with Retinal Consultants of San Diego. “In the past, we’ve relied on two-dimensional imaging to determine the presence or absence of fluid leaking from these abnormal vessels in the retina, and whether or not additional treatment is necessary with VEGF inhibitor drugs.
“My personal evaluation of AngioAnalytics suggests we now have another way to monitor or determine if additional treatment is necessary by documenting how the drug affects a specific CNV membrane in a specific patient, and how long that effect lasts,” added Dr. Tornambe.
AngioAnalytics brings objective data and analysis to Optovue’s commercially available AngioVue® OCTA technology that provides high-resolution imaging of retinal blood vessels. Combined, the two technologies create color-encoded maps of the vessel densities of the retina or optic nerve, and provide analyses of areas where there is blood vessel loss (non-perfusion), abnormal blood vessel growth (flow area), and several parameters to assess change to the foveal avascular zone, an area of the retina profoundly affected by diabetic retinopathy. The new AngioAnalytics software also provides trend analysis so that physicians can objectively monitor retinal and vascular changes caused by disease progression or from treatment.
Projection artifacts, inherent in OCTA technology from all manufacturers, occur when ghost images of blood vessels that exist in one retinal layer are projected onto other layers. The shadows from these vessels make it difficult to accurately interpret whether, and how much, abnormal vessel growth is actually present in the specific layers. 3D PAR technology is of paramount importance to improving OCTA image quality, as it forms the basis for accurate qualitative image interpretation and reliable measurements that enable physicians to quickly document disease states and incorporate OCTA technology into their diagnostic armamentarium.
“We are thrilled to receive marketing clearance for our innovative AngioAnalytics technology as part of the AngioVue System to aid physicians in the early detection and management of sight-threatening eye diseases in the U.S.,” said Jay Wei, founder and chief executive officer of Optovue. “Analytic measurement tools will shift the treatment paradigm because they provide an objective measure of treatment efficacy, thereby enabling truly customized patient management and improved patient care.
“The information provided by AngioAnalytics may help to change the clinical management of diabetic retinopathy, age-related macular degeneration and other diseases that progressively cause blindness for millions of people. We will continue to advance AngioAnalytics as we believe quantification is the gateway to widespread and global adoption of OCTA,” continued Wei.
Optovue will showcase AngioAnalytics on the AngioVue Imaging System in Booth #100 at the upcoming American Society of Retina Specialists meeting, July 20-25 in Vancouver, Canada at the convention center. Dr. Tornambe will speak at a dinner symposium on July 21 from 6:00-8:00 pm about the profound impact this new technology has had in his daily clinical practice. The symposium will take place in room 121-122 at the convention center.
About Optovue
Optovue, Inc. is a privately held medical device company founded in 2003. Headquartered in Fremont, Calif., the company is dedicated to the advancement and commercialization of high-speed OCT and OCTA technology used to facilitate the diagnosis and management of eye diseases, many of which may lead to permanent blindness. Optovue is the first company to develop and commercialize the pioneering OCTA technology. To date, there are over 400 peer-reviewed publications detailing the AngioVue Imaging technology and clinical applications. The company has installed over 11,000 products worldwide. For more information, visit www.optovue.com.