LA ROCHELLE, France--(BUSINESS WIRE)--Regulatory News:
VALBIOTIS (Paris:ALVAL) (FR0013254851 – ALVAL / PEA/PME eligible [PEA-PME share savings plan for providing financial aid to SMEs]), a company that specializes in developing innovative nutrition solutions designed to prevent cardiometabolic diseases and to provide nutritional support for patients, today announces positive results for the active ingredient of VAL-63-NAFLD for non-alcoholic fatty liver, a risk factor for NASH (non-alcoholic steatohepatitis), and for the related metabolic parameters. These results were selected and presented during the annual congress of the International Diabetes Federation in Abu Dhabi (UAE) on December 5, 2017.
Sébastien Peltier, Chairman of the VALBIOTIS Board of Directors, comments: "The preclinical results obtained for non-alcoholic fatty liver are very exciting. They make VAL-63-NAFLD a serious contender on the world market for these hepatic conditions, and further reinforce our mission of offering effective products that can reduce risk factors for cardiometabolic diseases. With VAL-63-NAFLD, VALBIOTIS has a mature portfolio, including products that have all proven their high potential and are in the clinical development phase."
> Non-alcoholic fatty liver: a risk factor for NASH with incidence numbers rising sharply worldwide
Metabolic liver diseases are closely linked to the development of type 2 diabetes and obesity, and are becoming a major public health issue worldwide. Non-alcoholic fatty liver (NAFL) is directly linked to diet and lifestyle, and is characterized by the accumulation of a large excess of triglycerides in the liver. It now affects 20% to 26% of the European population1 and 20% to 46% of the US population2.
This "fatty liver" is the first stage of a pathological process that evolves into non-alcoholic steatohepatitis (NASH), occurring in 30% of subjects with steatosis2. The prognosis is often poor (38% survival rate over a ten-year period) since NASH develops into fibrosis, then into cirrhosis, hepatic failure, and, in some cases, hepatocellular carcinoma3.
According to the World Gastroenterology Organisation, fatty liver and NASH are now the number one cause of hepatic disease in Western countries. Their prevalence has doubled over the last 20 years3. NASH is now one of the most common indications for a liver transplant. To date, there is no therapeutic or preventive solution on the market that has been proven to be effective.
> Hepatic triglycerides level, a NASH risk factor, is reduced by 40% in preclinical studies with TOTUM-63, the active ingredient of VAL-63-NAFLD
VAL-63-NAFLD, a product candidate containing active ingredient TOTUM-63 combined with a nutritional substance, is positioned for reducing non-alcoholic fatty liver, a risk factor for NASH.
In clinical studies, fatty liver is defined by a ratio of hepatocytes accumulating excess lipids3 greater than 5%. The level of hepatic triglycerides, a key parameter in the pathological process, is therefore a priority target for a product candidate designed to reduce the risk of developing NASH.
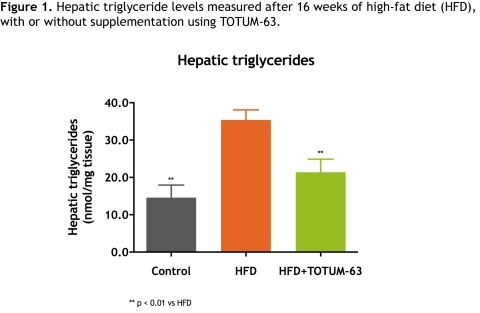
Preclinical studies conducted in C57BL6/J murine models kept on a high-fat diet showed a marked reduction of close to 40% in the level of hepatic triglycerides after 16 weeks of supplementation with TOTUM-63 (Fig. 1).
Figure 1. Hepatic triglycerides level measured after 16 weeks of high-fat diet (HFD), with or without supplementation using TOTUM-63.
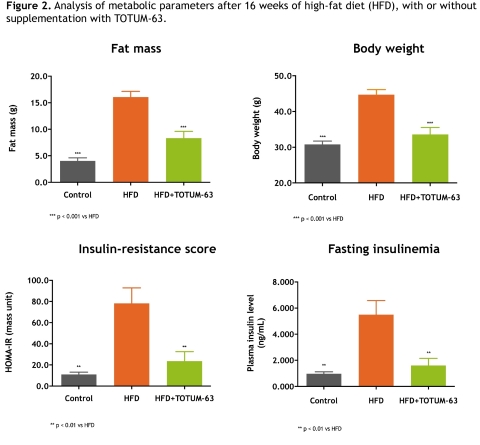
Moreover, the complete analysis revealed a very clear positive effect on two major metabolic determinants of non-alcoholic fatty liver: body fat mass and insulin resistance. Adiposity was thus reduced by 48.4% and total body weight by 24.8% after 16 weeks of supplementation. From an endocrinological point of view, peripheral insulin resistance, measured by the HOMA-IR score, was reduced by 69.9% and was logically associated with a 70.9% decrease in insulinemia, suggesting a pancreas-sparing effect and an improvement in glucose homeostasis (Fig. 2).
Figure 2. Analysis of metabolic parameters after 16 weeks of high-fat diet (HFD), with or without supplementation with TOTUM-63.
This whole preclinical data shows evidence of serious overall efficacy of the active ingredient of VAL-63-NAFLD on lipid metabolism, with a major positive physiological effect on the liver and its triglyceride content.
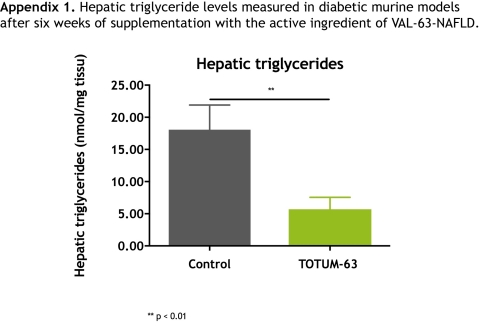
> A confirmation of the 68% reduction in hepatic triglycerides already obtained in the diabetic murine models*
Closely linked to type 2 diabetes or to insulin-resistance, non-alcoholic fatty liver is a complication which occurs in close to two-thirds of type 2 diabetics4.
Priorly, it was logical to test the active ingredient of VAL-63-NAFLD specifically on diabetic murine models (db/db) during preclinical phases, over a six-week period.
The results obtained in these models were similar, and even superior: TOTUM-63, the active ingredient of VAL-63-NAFLD had thus reduced the hepatic triglycerides level by 68.3% after 6 weeks of supplementation and had shown significant effect on body fat mass. Fasting blood sugar was also sharply decreased (cf. Appendix).
This data is particularly positive and confirms the very high efficacy potential of VAL-63-NAFLD to reduce non-alcoholic fatty liver, a risk factor for developing NASH. The data validates the implementation of a development plan for this product, which will be the subject of a clinical trial in the target population, in patients with non-alcoholic fatty liver.
ABOUT VALBIOTIS
VALBIOTIS specializes in developing innovative nutrition solutions designed to prevent cardiometabolic diseases and provide nutritional support for patients. Its products are made for manufacturers in the agri-food and pharmaceutical industries. VALBIOTIS particularly focuses on solutions to prevent type 2 diabetes, NASH (nonalcoholic steatohepatitis), obesity and cardiovascular diseases.
VALBIOTIS was founded in La Rochelle in early 2014 and has formed numerous partnerships with top academic centers in France and abroad, including the La Rochelle University, the CNRS and the Clermont Auvergne University located in Clermont-Ferrand, where the company opened a second office. These agreements enable it to benefit from a considerable leverage effect since it was set up thanks, in particular, to the experts and technical partners mobilized for these projects. VALBIOTIS is a member of the “BPI Excellence” network and received the “Innovative Company” status accorded by BPI France. VALBIOTIS has also been awarded “Young Innovative Company” status and has received major financial support from the European Union for its research programs by obtaining support from the European Regional Development Fund (ERDF).
Find out more about VALBIOTIS:
http://valbiotis.com/
Name: Valbiotis
ISIN code: FR0013254851
Mnemonic Code:
ALVAL
* Overview of data presented in the VALBIOTIS core document, registered on 5 April 2017 by the AMF, the French Financial Markets Regulator.
1 Younossi et al. Global epidemiology of nonalcoholic
fatty liver disease-Meta-analytic assessment of prevalence, incidence,
and outcomes, Hepatology, 2016
2 Williams et al.,
Prevalence of nonalcoholic fatty liver disease and nonalcoholic
steatohepatitis among a largely middle-aged population utilizing
ultrasound and liver biopsy: a prospective study, Gastroenterology, 2011
3
World Gastroenterology Organisation Global Guidelines. Non-alcoholic
Fatty Liver Disease and Non-alcoholic Steatohepatitis. http://www.worldgastroenterology.org/assets/export/userfiles/2012_NASH%20and%20NAFLD_Final_long.pdf
4
Loomba R et coll. Non-invasive screening of diabetics in primary care
for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther,
2015