BOSTON--(BUSINESS WIRE)--121nexus, a leader in utilizing image recognition technology to provide a new solution to the challenge of master data management (big data), appoints three recognized leaders in technology and medical device to a newly formed Advisory Board. The advisors, Doug Schmidt, Jay Crowley, and Marc Miller, will offer growth support to the company’s leaders and Board of Directors.
Says 121nexus CEO Charlie Kim, “The expertise of Doug, Jay, and Marc will help us as we continue to expand our reach in educating enterprise manufacturers, especially those in the medical device and pharmaceutical industries, about the value of the 121nexus platform.” He adds, “Bridging the technological gap between data sources, and turning the conventional data model on its head by using individual units for data validation, our platform has already proven that it saves companies valuable time and money.”
Doug Schmidt is Chief Information Officer at Dentsu Aegis Network where he leads Technology in the Americas for this top 3 public global media and advertising company. Schmidt previously was Senior Vice President, Chief Enterprise Architect, and Chief Information Security Officer at Pearson PLC (Pearson Education, Financial Times, Penguin Books) and prior to that was Vice President of Technology at CBS Interactive, Inc. (formerly CNET Networks). He also serves as an Advisor to other technology start-ups including VektorTek/SiLO, SerialBox.com, and SkootandDoodle.com.
Jay Crowley is former Senior Advisor for Patient Safety in the Food and Drug Administration’s (FDA) Center for Devices and Radiological Health, where he developed the framework and authored key requirements for the FDA’s new Unique Device Identification (UDI) system. Crowley held a variety of positions over his nearly 27 years at the FDA, including work with design control regulations to reduce the chance of human errors with medical devices, patient safety and adverse event reporting. Crowley also worked in the Office of the Commissioner of the FDA, and the Office of Compliance at the FDA. He is currently the Vice President and UDI Practice Lead at USDM Life Sciences, a leading global professional services firm focused on increasing the capacity of Life Science and Healthcare organizations to accelerate innovation and maximize productivity.
Marc Miller, a medical device global compliance expert, is the Division President of TransPerfect Medical Device Solutions, the specialized medical device division of TransPerfect. With annual revenues of more than $500 million, TransPerfect is the world's largest provider of language services and content management technology to the medical device industry. He was the CEO of Crimson Life Sciences which became TransPerfect Medical Device Solutions.
About 121nexus
Headquartered in Boston, MA, 121nexus
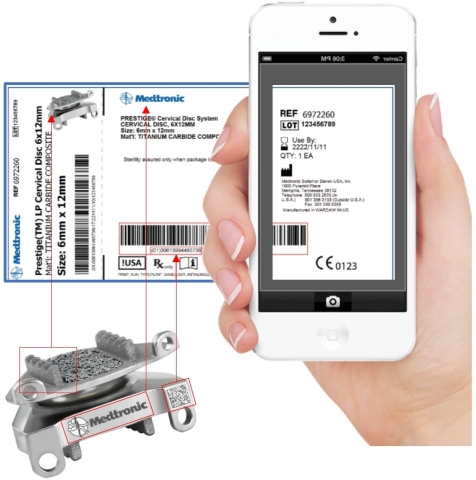
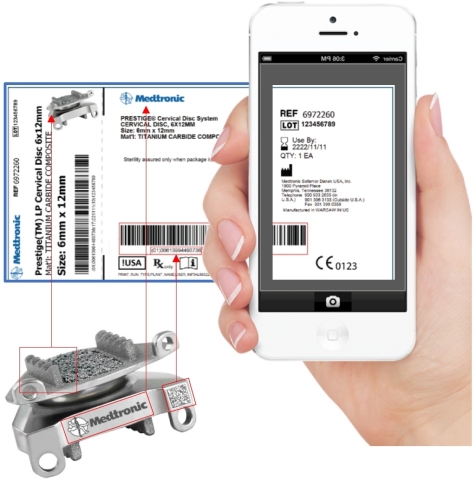
provides a cloud-based solution and an image recognition app that answer
the challenges of master data management (big data). For medical device
and pharmaceutical companies, 121nexus is a complete Unique
Device Identification (UDI) management solution.