COPENHAGEN, Denmark--(BUSINESS WIRE)--Non-surgical photorefractive intrastromal corneal collagen cross-linking, or PiXL, is a promising new treatment for vision improvement for low myopia, according to the results of two studies announced today at the XXXIV Congress of the European Society of Cataract and Refractive Surgeons (ESCRS).
“PiXL is a groundbreaking, first-in-category development in the field of vision improvement,” said Rajesh K. Rajpal, MD, Chief Medical Officer, Avedro. “We are thrilled with the progress in our clinical development and believe that PiXL represents a new non-invasive treatment paradigm for patients with low myopia. We are pleased to see these very promising early results.”
The first study, a prospective clinical trial conducted at Ruhr University Eye Hospital, Bochum, Germany, evaluated the safety and efficacy of epithelium-off PiXL. This 12 month study included 39 eyes (phakic and pseudophakic) in 2 groups (group 1: MRSE -1.0 D to -2.0 D, cyl. max. -0.75 D; group 2: MRSE -2.0 D to -3.0 D, cyl. max. -0.75 D).
Preliminary results at 12 months demonstrate significant improvement with mean uncorrected visual acutiy (UCVA) of 20/20 at 12 months, and no loss of best-corrected visual acuity (BCVA). Based on these early results, the data demonstrate that PiXL is a promising refractive procedure for low myopia, offering the potential for vision improvement without tissue ablation.
“The preliminary 12 month results build upon what we observed at the three and six month evaluations,” said Dr. Matthias Elling, FEBO, Ruhr University Eye Hospital, Bochum, Germany. “Based on these outcomes, the long-term stability of PiXL treatment appears favorable. It is exciting to see the potential of this new procedure for the large numbers of patients currently wearing contact lenses who have chosen not to have traditional refractive surgery.”
The second study, performed at Eagle Eye Centre in Singapore, designed to analyze the safety and efficacy of trans-epithelial PiXL in correcting low myopia, evaluated treatment results in 8 patients (14 myopic eyes, -1.62 ± 0.6D, range -0.75 to -2.625D) who underwent the procedure and were followed for 9 months postoperatively. Efficacy findings included a mean myopia reduction of 0.75D at 9 months. Safety evaulations found no significant loss in endothelial cell count or BCVA, and no significant corneal haze post-procedure. A questionnaire administered at 6 months post-op also found high levels of patient satisfaction.
“In addition to the positive safety and efficacy findings, we were pleased to find a high satisfaction rate for the procedure with patients,” said Dr. Lim Wee Kiak of Eagle Eye Centre in Singapore. “Current refractive procedures are invasive and weaken the cornea. PiXL is revolutionary in that it improves the refractive error by stiffening the cornea, while also being non-invasive. This technology has huge potential as an addition to our armamentarium for refractive corrections.”
About PiXL
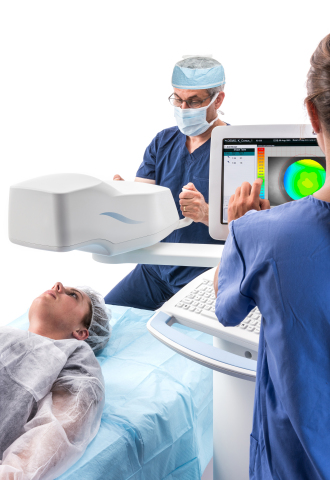
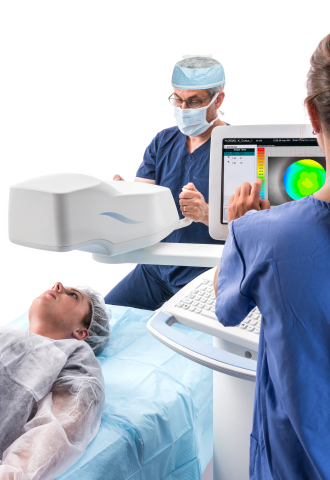
Avedro’s PiXL procedure offers the potential to provide non-invasive vision improvement for low myopia without compromising corneal biomechanical integrity. PiXL treats myopic refractive errors through the topical application of riboflavin followed by exposure of the cornea to UVA light delivered by the Mosaic device, which uses advanced eye-tracking technology in order to deliver controlled cross-linking.
The Mosaic device has received both the CE mark in Europe, and approval from Health Canada. The PiXL procedure and Mosaic device are not available in the United States.
About Avedro, Inc.
Avedro is a privately held pharmaceutical and medical device company advancing the science and technology of corneal cross-linking and refractive correction.
Avedro’s Photrexa® Viscous, Photrexa® and KXL® products are approved for sale in the United States. Avedro’s products sold outside of the United States include capital equipment such as the UV-X devices, the KXL and Mosaic™ Systems, and related proprietary pharmaceuticals such as the VibeX® and MedioCROSS® formulations. Avedro distributes its products in countries outside of the United States through a network of ophthalmic medical device distributors.