SALT LAKE CITY--(BUSINESS WIRE)--Q Therapeutics, Inc., a clinical-stage developer of novel cellular therapies for central nervous system (CNS) diseases, announced today that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug Application (IND) for the initiation of Phase 1/2a clinical trials of its Q-Cells® product in patients with amyotrophic lateral sclerosis (ALS). Also known as Lou Gehrig’s disease, ALS is a devastating condition caused by degeneration of motor neurons, the nerve cells in the brain and spinal cord that control muscle movement. ALS affects more than 30,000 people in the U.S. at any given time and nearly half a million people worldwide. There is no effective therapy for ALS and it is 80% fatal within five years of diagnosis.
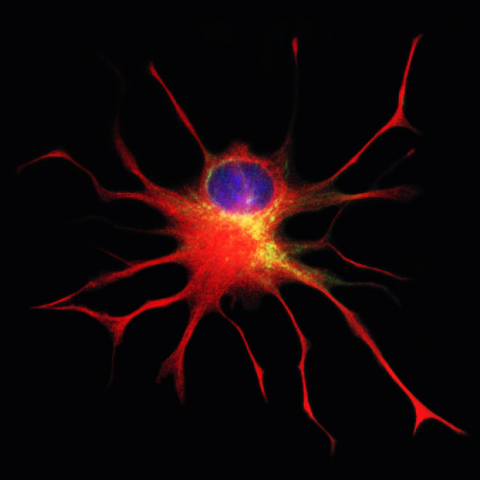
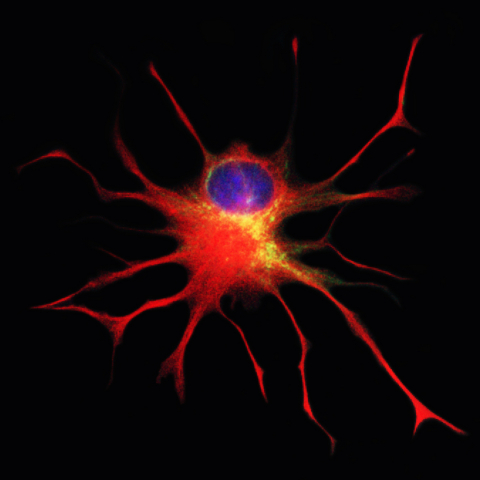
Q-Cells, the Company’s first patented cellular therapeutic product candidate, are glial-restricted progenitor cells (GRPs) – early descendants of neural stem cells that produce only “glia” – which make up 50 percent of cells in the brain. Glia are essential for supporting, maintaining and even restoring neuron health. The FDA’s clearance of Q Therapeutics’ Investigational New Drug (IND) application for Phase 1/2a clinical trials with Q-Cells in ALS patients confirms that the preclinical data are sufficient to allow initiation of the first-ever human trials evaluating the use of GRPs to treat ALS patients. Published pre-clinical data in animal models of ALS and other central nervous system (CNS) diseases have demonstrated that delivery of healthy glial cells into the brain and spinal cord has the potential to modify the course of currently incurable CNS diseases and injuries.
“This is a major milestone not only for Q Therapeutics but also in the quest for a cure for ALS. Our unique approach holds great promise and we look forward to the potential of these trials to establish human safety and efficacy,” said Deborah Eppstein, Ph.D., President and Chief Executive Officer of Q Therapeutics. “This achievement builds on a decade of research, nearly $30 million of funding, and the tireless dedication of our scientists and research collaborators. We proudly acknowledge the devotion of all of our scientific co-workers and supporters, especially James Campanelli, Ph.D.; Mahendra Rao, MD, Ph.D.; the National Institutes of Health; the Maryland Stem Cell Research Fund; and Bosarge Life Sciences, in the pursuit of novel therapies for ALS and other debilitating diseases of the brain and spinal cord.”
“Every 90 minutes someone in the world is diagnosed with ALS, and current therapies offer little hope,” said James Campanelli, Ph.D., Vice President of Research and Development at Q Therapeutics. “With this FDA clearance, we are on the cusp of performing groundbreaking human studies with a cellular therapeutic that has the potential to change the outlook for people living with ALS and their loved ones. The FDA’s clearance of our IND for Q-Cells within the original 30-day review period provides regulatory validation of our careful development approach and clinical trial plans.”
Mahendra Rao, MD, Ph.D., Chief Strategy Officer at Q Therapeutics, said, "GRP cells are really a unique clinical candidate and these will be the first ever human trials. What is special is that their lineage restriction means that after transplantation they only generate oligodendrocytes and astrocytes in vivo – vital new glial cells – and, unlike many other cells, they migrate, survive and respond to cellular signals to produce therapeutic molecules after injury. We are hopeful that these progenitor characteristics of the GRP will prove effective in the treatment of a variety of CNS disorders where repair of the cellular machinery of the brain and spine is required."
The FDA has previously granted Q-Cells orphan drug status for ALS, a designation intended to advance the evaluation and development of therapeutics that demonstrate promise for the treatment of rare diseases. Q Therapeutics selected ALS as the first clinical indication for Q-Cells based on a combination of the large unmet medical need and the significant scientific rationale supporting the multiple pathways by which healthy glial cells are believed to protect and preserve the function of motor neurons. Q Therapeutics’ Phase 1/2a clinical trial, designed to evaluate the safety and tolerability of Q-Cells in ALS patients, will begin once Institutional Review Board (IRB) approval has been obtained and funding secured.
In collaboration with Nicholas Maragakis, MD, at Johns Hopkins University, Q Therapeutics has received considerable support from the National Institute of Neurological Disorders and Stroke Translational Research Program (U01-NS06713) for the preclinical development of Q-Cells. Q Therapeutics worked with MPI Research, Inc., to carry out animal safety studies, with Goodwin Biotechnology, Inc., to manufacture the antibody used in cell purification, and with the Biologics Consulting Group, for regulatory guidance. The Company is also partnering with the Cell Therapy and Regenerative Medicine Facility (CTRM) at the University of Utah to manufacture Q-Cells for the upcoming clinical trial.
About Q Therapeutics, Inc. – Headquartered in Salt Lake City, Q Therapeutics is a fully reporting, non-trading clinical-stage company developing adult stem cell therapies to treat debilitating diseases and injuries of the central nervous system. The Company’s first product candidate, Q-Cells®, is a cell-based therapeutic intended to restore or preserve normal activity of neurons by providing essential support functions that occur in healthy central nervous system tissues. Q-Cells may be applicable to a wide range of central nervous system diseases, including demyelinating conditions such as multiple sclerosis, transverse myelitis, cerebral palsy and stroke, as well as other neurodegenerative diseases and injuries, such as ALS (Lou Gehrig’s disease), Huntington’s disease, spinal cord injury, stroke, traumatic brain injury, Parkinson’s disease and Alzheimer’s disease. Q Therapeutics’ initial clinical target is ALS, with a first IND now cleared to proceed by the FDA. The Company’s proprietary product pipeline also encompasses neural cell products derived from induced pluripotent stem cells (iPSC). For more information, please visit www.qthera.com.
Cautionary Statement Regarding Forward-Looking Information – This news release may contain forward-looking statements made pursuant to the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that such forward-looking statements in this press release regarding potential applications of Q Therapeutics’ technologies constitute forward-looking statements that involve risks and uncertainties, including, without limitation, risks inherent in the development and commercialization of potential products, uncertainty of clinical trial results or regulatory approvals or clearances, need for future capital, dependence upon collaborators, and maintenance of its intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements. Additional information on potential factors that could affect results and other risks and uncertainties are detailed from time to time in Q Therapeutics’ periodic reports, including the quarterly report on Form 10-Q for the period ended March 31, 2015, and the Company’s Annual Report on Form 10-K for the year ended December 31, 2014.