SAN DIEGO--(BUSINESS WIRE)--New research presented Sunday at the 34th Annual meeting of the International Society for Heart and Lung Transplantation (ISHLT) demonstrated the CorMatrix particulate extracellular matrix (P-ECM) alone and as an adjunct to HeartWare International Inc.’s left ventricular assist device (LVAD) improved cardiac function in a bovine ischemic heart failure model, as compared to LVAD alone. Mark S. Slaughter, M.D., of the Department of Cardiovascular and Thoracic Surgery and his research team from the University of Louisville, Louisville, KY, presented data during the ISHLT Mechanical Circulatory Support: Bench to Bedside Session in his presentation entitled “Myocardial Regeneration and Recovery with Extracellular Matrix and LVAD Support” on Sunday Morning.
This first of its kind pre-clinical study was designed to evaluate if combining a LVAD with an extracellular matrix bioscaffold therapy would be more effective at improving cardiac function in ischemic heart failure than either therapy alone. LVAD’s serve as a bridge to heart transplantation or to assist the mechanical function for patients with advanced stage heart failure, whereas the P-ECM facilitates native cardiac progenitor cell engraftment and proliferation resulting in positive remodeling of the damaged heart muscle. The combined effects could encourage endogenous repair mechanisms to take hold, reversing the typical downward spiral patients experience following ischemic events.
According to Dr. Slaughter “combining P-ECM and LVAD therapies may synergistically provide a favorable environment to enhance myocardial regeneration and promote sustained myocardial recovery and lead to LVAD removal which may improve patient outcomes and reduce healthcare costs.”
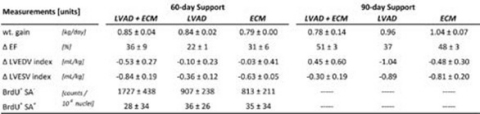
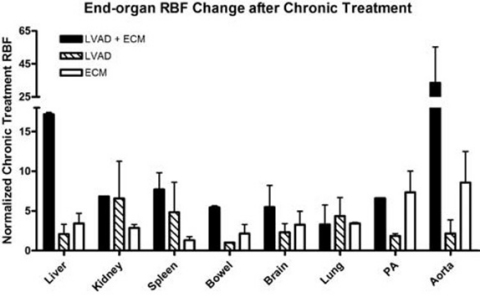
Ejection Fraction (EF) increased 42% with LVAD+ECM (33±9% to 75±2%), 40% with P-ECM (27±3% to 67±12%), and 27% with LVAD (38±7% to 65±7%). LVAD+ECM demonstrated the largest increase in cell proliferation (Fig 1). Additionally, LVAD+ECM resulted in the highest end-organ RBF (Fig 2).
“CorMatrix is very encouraged with the results of this animal study, which confirm our belief that P-ECM will play a vital role in treating patients with ischemic heart failure. The results demonstrated the safety of the P-ECM and the potential for it to improve cardiac function, cardiac and end organ perfusion, and repopulate the damaged myocardium with new, functioning tissue," said Robert Matheny, Chief Medical Officer, CorMatrix Cardiovascular.
Based on this study and other pre-clinical studies, CorMatrix plans to initiate treatments in a First in Human study at the Central Clinical Hospital, Warsaw, Poland in the second quarter of 2014 with principle investigator Piotr Suwalskl, M.D., Chief of the Department of Cardiac Surgery and Professor of Cardiac Surgery.
Background of Extracellular Matrix (ECM) Biomaterial
The decellularized matrix material serves as a bioscaffold to allow vascular ingrowth from adjacent tissues to deliver progenitor cells and nutrients to the matrix, which then differentiate into tissue-specific cells and structures. The ECM material is gradually replaced as the patient’s own cells reinforces and rebuilds the diseased or damaged site. During repair, the matrix is naturally degraded and resorbed, leaving remodeled functional tissue where damaged or injured tissue would normally be expected. The safety of extracellular matrices has been well established in a number of different clinical applications and more than 500 published papers. Since 1999, an estimated two million patients worldwide have received an extracellular matrix implant.
About CorMatrix
CorMatrix® Cardiovascular, Inc. was founded in 2001 as a privately held medical device company dedicated to developing and delivering innovative biomaterial devices that harness the body’s own innate ability to repair damaged cardiovascular tissues. Headquartered in Atlanta, Georgia, the company is currently researching, developing and commercializing a platform technology known as CorMatrix ECM® for a variety of cardiovascular indications, and has U.S. clearance and European approval (with a CE Mark) for its ECM® technology as an implant for pericardial closure, cardiac tissue repair, as well as clearance for carotid repair. With significant patent protection, CorMatrix is poised to successfully expand its current line of products. For more information, visit www.cormatrix.com.
Safe Harbor Statement
This press release includes statements that look forward in time or that express management's beliefs, expectations or hopes. Such statements are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, the anticipated approval of pending and future patent applications related to CorMatrix® ECM® Technology, the Company's future patent application filings and the protection of the Company's intellectual property provided by any patents that issue. These statements are based on current information and belief, and are not guarantees of future performance. Among the risks and uncertainties that could cause actual results to differ materially from those indicated by such forward-looking statements include that pending and future patent applications related to CorMatrix® ECM® Technology may not result in issued patent, that the issuance of any patents may be delayed, that the allowed claims, if any, may not be in line with the Company's expectations, that the Company may not be successful in enforcing its patents, and the risk factors detailed from time to time in the Company's periodic Securities and Exchange Commission filings, including, without limitation, its 10-K filing for the fiscal year ended December 31, 2012. By making these forward-looking statements, the Company does not undertake to update them in any manner except as may be required by the Company's disclosure obligations in filings it makes with the Securities and Exchange Commission under the federal securities laws.