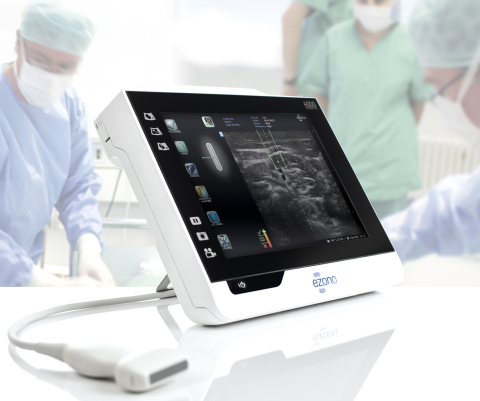
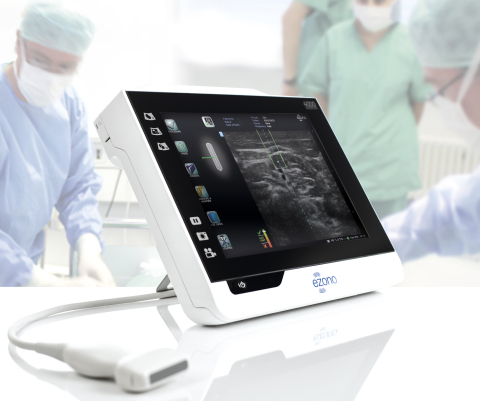
JENA, Germany--(BUSINESS WIRE)--eZono AG (“eZono”), a private corporation designing, developing and distributing state of the art tablet ultrasound systems, today announced it received 510(k) clearance from the FDA for its eZono 4000 dedicated procedural guidance system with eZGuide technology, providing any plane freehand navigation.
The eZono 4000 offers excellent image quality and features eZGuide, a revolutionary needle guidance providing needle tip location, with the precision and ease of use that clinicians did not think possible in a tablet style system. “The eZono 4000 with eZGuide, is exceeding our expectations in performance and has been welcomed as game changing technology by many physicians”, says Graham Cox, CEO eZono. “eZono is passionate about bringing practical and affordable solutions to clinicians in their everyday practice and we are delighted to bring this technology to the United States for needle guidance applications. As healthcare costs rise, it becomes imperative for companies to provide quality solutions at affordable prices. eZono addresses the needs of improving patient safety with increased efficiency by making essential equipment more easily accessible with minimal ongoing costs per procedure”.
“The eZono 4000 has revolutionized the way in which ultrasound guided procedures are performed in two ways” says Dr Jeff Gadsden, Director of Regional Anesthesia St. Luke's-Roosevelt Hospital Center, New York “Firstly, knowing where your needle tip is at all times represents a substantial safety advantage, both for in-plane and out-of-plane procedures. The needle localization technology eliminates the guesswork out of ultrasound guided techniques and reassures you that the procedure is not going to end in misadventure. Secondly, we have found the learning curve is accelerated with the eZono 4000 -- trainees understand ultrasound guided block techniques more quickly when they are trained on the 4000 and are able to demonstrate better hand-eye coordination skills after several sessions with the machine compared to traditional teaching techniques”.
About eZono
eZono AG (www.eZono.com) was founded in 2004 and is headquartered in Jena, Germany. eZono is a young dynamic company focused on designing, developing and distributing point of care solutions. Specializing in procedural applications, eZono brings a diverse and fresh approach to ultrasound guidance with innovative and unique solutions. The company is dedicated to supplying sophisticated solutions to address the real needs for point of care clinicians offering state of the art education, image quality and precision for needle guidance in one system. Visit www.ezono.com for more information.