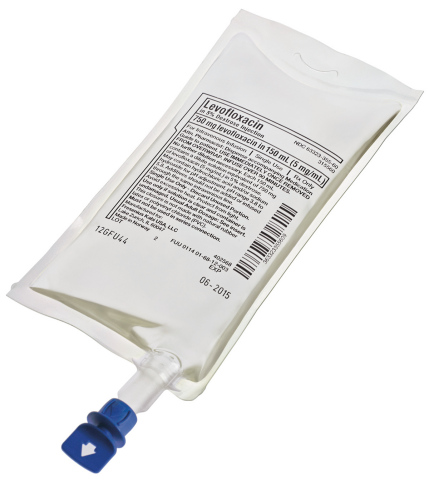
LAKE ZURICH, Ill.--(BUSINESS WIRE)--Fresenius Kabi USA announced today the availability of Levofloxacin in 5% Dextrose Injection in freeflex® containers.
Freeflex® is an innovative container designed for infusion solutions that has been marketed globally since 2005 and in the U.S. since 2008. Freeflex’s multilayer polyolefin film is PVC-free, plasticizer-free, non-DEHP and designed for easy handling with enhanced safety, environmental performance in mind.
“The introduction of Levofloxacin using the freeflex technology expands our market leading anti-infective business with a ready to use formulation and provides customers with a new choice in serving their patients,” said John Ducker, president and CEO of Fresenius Kabi USA.
Levofloxacin is a fluoroquinolone antibacterial indicated for use in the treatment of bacterial infections caused by susceptible bacteria, including pneumonia, bronchitis, skin infections, urinary tract infections, prostatitis and anthrax. Fresenius Kabi USA received FDA approval for Levofloxacin in 5% Dextrose Injection in June, 2013.
According to IMS, in 2012 the total U.S. market for Levofloxacin premix was $69 million.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com) is a leading global health care company that focuses on pharmaceuticals and medical devices used to care for critically and chronically ill patients inside and outside the hospital. The company’s products include intravenous specialty and generic medicines, infusion therapies, clinical nutrition and related medical devices. Fresenius Kabi expanded its presence in the United States in 2008 with the acquisition of APP Pharmaceuticals, Inc. and again in 2012 with the acquisition of Fenwal Inc. The company's roots go back more than 100 years to the founding of Fresenius SE, in Bad Homburg, Germany, where the company is headquartered today.
In the United States, APP, a division of Fresenius Kabi USA, LLC markets a broad portfolio of injectable and specialty pharmaceutical products with a focus on the oncology, anti-infective, anesthetic/analgesic and critical care markets for use in hospitals, long-term care facilities, alternate care sites and clinics. For more information, visit www.APPpharma.com.
Forward Looking Statement
To the extent this release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.