COLOGNE, Germany--(BUSINESS WIRE)--Roche and Ncardia have reached a broad licensing agreement whereby Roche and its affiliate companies gain access to key disease modeling patents from Ncardia.
Ncardia was recently formed through the merger of Axiogenesis and Pluriomics. The two companies have pioneered the development of stem cell derived cells for drug discovery and safety and in particular have advanced disease and tissue modeling applications.
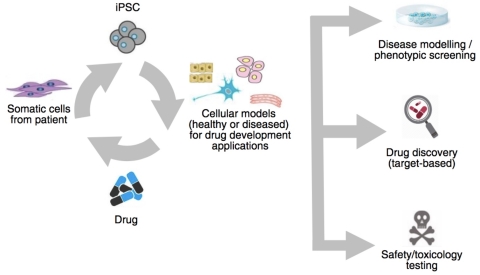
The Ncardia patent portfolio consists of several granted patents in Europe, the USA and Japan. The drug screening and disease modeling technology not only involves patient iPSC cells, induction of the disease phenotype can also be achieved through genetic manipulation, mRNA or siRNA transfection or through chemical or physical induction.
Dr Stefan Braam, CEO Ncardia, commented: “Ncardia mission is to support and enable our clients to make better medicines faster. The combination of iPSC derived disease models with phenotypic screening is an extremely powerful method to identify new drug candidates. This license marks a further step in Ncardia technology strategy aimed at addressing the potential of stem cell applications in the drug discovery and development market."
Roche has now obtained a non-exclusive, worldwide license to the key Ncardia disease modeling patents claiming the use of stem cell derived cells disease models for the discovery & development of novel drug candidates. The financial terms of the agreements are not disclosed.
“This license is the first step of our strategy to make our technology available to the pharmaceutical drug discovery & development market.” Felix von Haniel VP Sales and BD of Ncardia. In addition to disease modeling applications Ncardia is committed to providing the most predictive healthy human cellular models to improve and accelerate safety testing.
Ncardia: Ncardia (www.ncardia.com) believes that stem cell technology will help to get better medicines to patients faster. The company develops, produces and commercializes highly predictive human cellular assay systems for safety and efficacy testing. The cardiac product portfolio encompasses a broad panel of hiPSC derived Cardiomyocytes (Pluricyte®, Cor.4U® and vCor.4U™) as well cardiac fibroblasts (FibroCor.4U™). In addition, the company delivers the Cardioplate™ product line of quality controlled ready to use assay plates. The neural cell portfolio contains the pan-neuronal product CNS.4U®, the peripheral neurons product Peri.4U®, and astrocyte product Astro.4U®.
Ncardia is committed to deliver its clients working assays solutions through in house assay development and extensive support. The company has a wide range of supported applications and compound assay services such as of electrophysiology, biochemistry and contraction based assays for predictive safety pharmacology and toxicology testing. In addition, Ncardia develops and provides its customers with a broad portfolio of cardiovascular services from disease modeling to cardiovascular drug efficacy screening.
Ncardia is based in Belgium, the Netherlands, Germany and in the USA. The company is privately held and established following the merger of Pluriomics and Axiogenesis.