BOSTON--(BUSINESS WIRE)--Etiometry, the leader in clinical decision-support software, announced today its IVCO2 Index™ was cleared by the Food and Drug Administration for use with neonatal ICU patients under 2kg, who are especially vulnerable to the effects of hypercapnia. This is Etiometry’s 9th FDA clearance and its second this year.
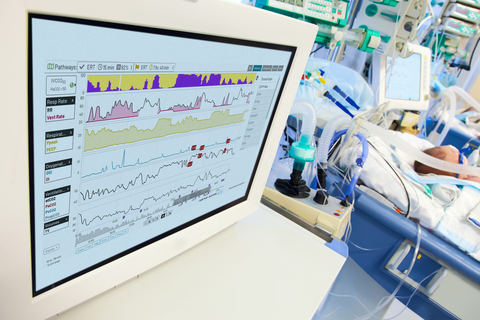
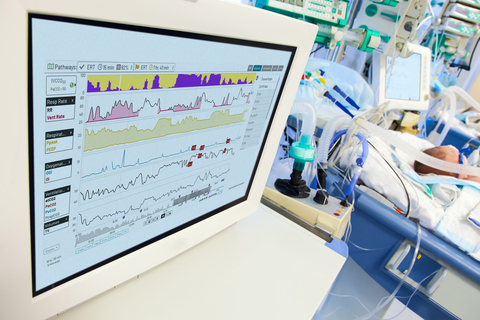
Etiometry’s IVCO2 Index™ is a game changer in critical care, seamlessly integrating multiple data sources to provide an individualized assessment for ventilation-perfusion mismatch and detect the likelihood a patient will have a PaCO2 of greater than 50 mmHg in mechanically ventilated patients. It has been specifically designed to overcome the challenges of existing conventional monitoring, such as arterial blood gas testing and transcutaneous monitoring for tiny premature newborns.
“The IVCO2 Index™ is a first-of-its-kind algorithm that increases the ability to detect hypercapnia risk without needing additional hardware attached to these fragile patients,” said Dimitar Baronov, CTO of Etiometry. “It allows clinicians to prioritize care for patients who need it most.”
Many NICU patients require ventilatory support and their conditions can change quickly. Vigilant monitoring for hypercapnia is critical for maximizing neurodevelopmental outcomes and preventing complications. Digital monitoring illuminates the patient's condition between routine blood gas testing, adding another layer of protection to prevent oversights that may lead to poor outcomes.
“Our proprietary algorithm’s accurate and continuous monitoring for hypercapnia will enable the expansion of improved outcomes achieved by Etiometry partners within cardiac ICUs and PICUs into the NICU environment,” said Shane Cooke, CEO of Etiometry.
Etiometry will showcase all of its risk indices and ways the platform helps improve outcomes by informing escalation and de-escalation decision-making at the upcoming World Congress of Pediatric Cardiology and Cardiac Surgery in Washington, D.C. later this month. Etiometry also supported the submission of five abstracts for this event.
About Etiometry
Founded in 2010, Etiometry is the leader in clinical decision-support software designed to help clinicians in the intensive care setting make data-based decisions regarding their patients’ care and treatment. The company’s technologies provide valuable clinical insight and analysis to support early recognition of subtle changes in patients’ conditions to avoid complications and speed recovery. Etiometry has nine FDA clearances and four Health Canada approvals and CE markings. With roots in pediatric ICUs, Etiometry is utilized by some of the world’s top academic medical centers as well as leading children’s hospitals ranked by US News and World Report and Newsweek. Etiometry is committed to improving patient outcomes, increasing clinical efficiency, and lowering the cost of care through the more effective use of data.
The Etiometry Platform is the only critical care software solution that reveals deep insights into patient physiology, helping critical care teams deliver standardized and individualized care - and provides the ability to automate care escalation and de-escalation decisions. It is designed to facilitate the use of all available data to support the anticipation and management of the dynamic condition of patients requiring intensive care. To learn more, visit www.etiometry.com.