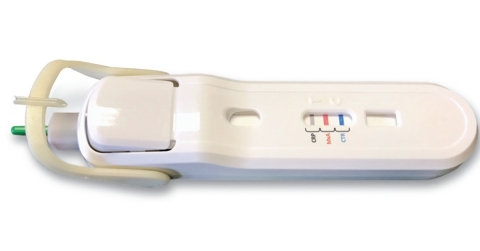
SARASOTA, Fla.--(BUSINESS WIRE)--RPS Diagnostics, Inc. (RPS®) today announced an updated CE mark of its FebriDx® test, clearing the way for its immediate launch in the European Union and all countries recognizing the CE mark. FebriDx is a rapid, accurate, and simple to use point-of-care test that is expected to contribute towards a diagnostic solution to the global antibiotic crisis.
FebriDx provides clinicians with a 10-minute assessment of the body’s immune response to an acute respiratory infection (ARI) directly from a fingerstick blood sample. The single use, disposable test identifies patients who have a clinically significant underlying infection and aids in the differentiation of viral and bacterial ARIs through the rapid detection of both Myxovirus resistance protein A (MxA) and C-reactive protein (CRP). MxA is an intracellular protein that becomes elevated in the presence of acute viral infection and CRP is a nonspecific inflammatory protein that is elevated in the presence of any clinically significant infection. Thus, unlike a standalone CRP test, MxA confers specificity to the test through the combined interpretation of the results.
The updated FebriDx test incorporates an all-in-one plastic housing technology that includes a built-in safety lancet, blood collection and delivery system, and integrated push button buffer delivery feature, which together, improve test convenience. The FebriDx test requires no additional equipment to perform or to interpret results. Through comprehensive analytical testing as well as a multicenter precision and reproducibility study, RPS Diagnostics successfully demonstrated equivalency to the previously CE marked version of the product. “Obtaining the updated CE mark triggers the initial international commercial launch of the FebriDx test,” said Robert Sambursky, MD, president and chief executive officer of RPS. “This test will provide a cost effective outpatient solution to improve antimicrobial stewardship.”
The timely FebriDx test results provide clinicians the ability to formulate a targeted clinical management and therapeutic decision plan during the initial patient encounter. Antibiotic misuse is a complex global problem that leads to antibiotic resistance, avoidable adverse events, and contributes to rising healthcare costs, largely driven by diagnostic uncertainty and patient pressure. More than 50% of all antibiotic prescriptions are unnecessary and are generated in the outpatient primary and urgent care setting. In a recent study in the United Kingdom, FebriDx was shown to alter clinical management decisions in 48% of patients tested, and to reduce unnecessary antibiotic prescriptions by 80%. Each day that goes by without the implementation of a cost effective solution is exacerbating the current global antibiotic crisis.
“The elegance of the FebriDx test lies in its simplicity,” said Robert Sambursky, MD. “It’s equally important not to miss treating an underlying bacterial infection as it is withholding unnecessary antibiotics from patients with clinically insignificant or viral infection. While most research and investment over the past decade has focused on rapid molecular testing to identify specific pathogens, FebriDx utilizes the body’s own immune system to provide a broad, fast, accurate, and cost-effective method to identify patients who may benefit from appropriate antibiotic therapy.”
For more information about the FebriDx test, visit FebriDx.com.
RPS Diagnostics
RPS Diagnostics (RPS) is an emerging developer, manufacturer, and marketer of cost-effective point-of-care (POC) tests for systemic infectious disease and antibiotic stewardship. The company’s innovative and patented FebriDx® test is a rapid, disposable, in-office test that uses a fingerstick blood sample to help identify a clinically significant immune response and differentiate bacterial from viral causes for fever in acute respiratory infection. With a 97% negative predictive value, FebriDx delivers results in 10 minutes and can be used to help triage infectious patients during the initial office visit, providing clinicians with actionable information that can be used to limit unnecessary antibiotics. At this time, the FebriDx test has not received U.S. Food and Drug Administration (FDA) clearance and is not commercially available in the United States. For more information on RPS and its products, visit RPSdetectors.com.