JERUSALEM--(BUSINESS WIRE)--Teva Pharmaceutical Industries Ltd. (NYSE & TASE:TEVA) announced today a new organization and leadership structure aimed to achieve better commercial focus and drive value creation. The new structure will enable strategic alignment across the portfolio, across regions and between functions, leveraging scale, enhancing agility, extracting efficiencies and providing increased proximity to the markets. This new structure will be implemented effective immediately.
Kåre Schultz, Teva’s President and CEO, said, “Teva is taking decisive and immediate action to address external pressures and internal inefficiencies. Our new company structure will enable stronger alignment and integration between R&D, operations and the commercial regions, allowing us to become a more agile, lean and profitable company."
Schultz continued, "We will focus on driving sustainable value creation. The new management team will position Teva for turnaround in the short to medium term. We are already working on a detailed restructuring plan for Teva and will share it in mid-December. It remains our absolute priority to stabilize the company’s operating profit and cash flow in order to improve our financial situation, while being focused on short-term revenue and cash generation, and at the same time, ensure we deliver on our commitment to supply high-quality medicines to patients around the world."
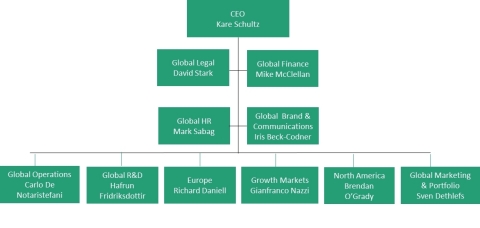
New structure
- The commercial business will no longer have two separate global groups for generics and specialty medicines, and will be integrated into one commercial organization, operating through three regions – North America, Europe and Growth Markets. Each of the regions will manage the entire portfolio – including generics, specialty and OTC - with full end-to-end P&L accountability. Some of the former global units will be integrated into the new structure, while others will be made redundant.
- The former Generic R&D and Specialty R&D organizations will be combined into one global group with overall responsibility for all R&D activities – generic, specialty and biologics – maximizing ROI through better focus and efficiency.
- A newly formed Marketing & Portfolio function will be responsible for overseeing the interface between regions, R&D and operations throughout all product lifecycle stages and optimizing generic and specialty portfolios across the therapeutic areas.
- The new structure will enhance alignment and seamless integration between Teva's Global Operations, the commercial regions, R&D and the Portfolio function, will increase productivity and simplify the organization.
- The commercial structure will rely on one leaner supporting organizational infrastructure that includes Finance, Legal, HR, and Global Brand & Communications.
As a result of these changes, Dr. Michael Hayden, Dr. Rob Koremans and Dipankar Bhattacharjee will retire from Teva, effective December 31, 2017.
New executive management team
Michael (Mike) McClellan is appointed Executive Vice President, Chief Financial Officer and will oversee the Finance Group, Business Development, Investor Relations and Information Technology. Previously, he served as Interim CFO and as SVP & CFO Global Specialty Medicines. Prior to joining Teva, Mike was the U.S. CFO at Sanofi.
Dr. Hafrun Fridriksdottir is appointed Executive Vice President, Global R&D. Previously, she served as President of Global Generics R&D. Prior to joining Teva, Hafrun served as Senior Vice President and President of Global Generics R&D in Allergan plc.
Brendan O'Grady is appointed Executive Vice President, North America Commercial. Brendan has previously served as Chief Commercial Officer, Global Specialty Medicine, as interim head of Teva’s European specialty business and as President and CEO for Teva’s North America generics business and as VP US Market Access and Reimbursement.
Richard Daniell is appointed Executive Vice President, European Commercial, after having served as President and CEO, Teva Generics Europe.
Gianfranco Nazzi is appointed Executive Vice President, Growth Markets Commercial. Gianfranco has previously served as President and CEO of Growth Markets at the Global Generic Medicines group, and prior to that he was he has served as Senior Vice President, Specialty Medicines Europe.
Sven Dethlefs is appointed Executive Vice President, Global Marketing & Portfolio. He previously served as Global Head of Respiratory Medicines and as Chief Operating Officer, Teva Global Operations.
All appointments are effective immediately, while the retiring executives will stay with Teva to support the transition until the end of the year.
The following members of Teva's executive management team will continue in their current positions:
Carlo de Notaristefani, Executive Vice President, Global Operations;
Iris Beck-Codner, Executive Vice President, Global Brand & Communications;
Mark Sabag, Executive Vice President, Global Human Resources;
David Stark, Executive Vice President, Chief Legal Officer.
Kåre Schultz concluded, "I would like to thank Dr. Michael Hayden, Dr. Rob Koremans and Dipankar Bhattacharjee for their profound contributions to Teva over the past decade and for their tireless dedication to the many patients we serve."
Bios of new appointments
Michael (Mike) McClellan has been serving as Teva Interim Group CFO since July 2017. Prior to this role, he had served as SVP & CFO Global Specialty Medicines since July 2015. Prior to joining Teva, Mike was the U.S. CFO at Sanofi, where his career spanned nearly 20 years in roles of increased responsibility in global finance and healthcare. Mr. McClellan received his BSBA, Accounting & Economics from the University of Missouri Trulaske College of Business.
Dr. Hafrun Fridriksdottir has been serving as Executive Vice President, President of Global Generics R&D since February 2017, after serving as Senior Vice President and President of Global Generics R&D from 2016. Prior to joining Teva, from 2015 to 2016, Ms. Fridriksdottir served as Senior Vice President and President of Global Generics R&D in Allergan plc. From 2002 to 2015, she held positions of increasing responsibility within the Actavis Group, including Senior Vice President, R&D. From 1997 to 2002, Ms. Fridriksdottir served as Divisional Manager of Development at Omega Pharma, until its merger with Actavis. Ms. Fridriksdottir started her career as a scientist for 2 years at a research and development company owned by Pharmacia in Sweden, after receiving an MS degree in pharmacy and a Ph.D. in physical pharmacy from the University of Iceland.
Brendan O’Grady has been serving as Chief Commercial Officer, Global Specialty Medicine division since Aug 2016. In addition, he currently serves as the interim head of Teva’s European Specialty business. Prior to these roles, Mr. O’Grady held the position of President and CEO for Teva’s North America generic business in 2015 and held various senior roles since he joined Teva in 2011 as Regional Account Manager. Prior to joining Teva, Mr. O’Grady spent 10 years with Sanofi predecessor companies in a variety of commercial and medical affairs roles that began in field sales. He received his B.S. from Geneseo State University, NY in Management Science/Marketing and holds an M.B.A. from Baker University in Baldwinsville, Kansas.
Gianfranco Nazzi has been serving as President & CEO of Growth Markets, Global Generic Medicines Group since March 2017. Mr. Nazzi joined Teva as Senior Vice President Specialty Medicines Europe in 2014. Prior to joining Teva, he served 7 years at AstraZeneca in various senior roles, including Sales and Marketing Vice President Europe, Global Vice President Respiratory, General Manager of the Balkans and Vice President Primary Care in Italy. At GlaxoSmithKline he served for two years as BU Director Metabolic & Cardiovascular and at Eli Lilly and Company he served for 5 years in various sales and marketing roles in both Italy and the US. He began his career at Danieli. Mr. Nazzi received his BA degree in economics from the University of Udine, and his Masters degree in Management Studies from SDA Bocconi.
Richard Daniell has been serving as President and Chief Executive Officer, Teva Generics Europe since Dec 2016. Prior to that, he had served as Chief Integration Officer Leading Actavis-Teva Integration since Sep 2015 after holding various senior roles, including Chief Operating Officer, Growth Markets and Regional General Manager for Teva in the UK and Ireland from 2012 through 2014. Mr. Daniell joined Teva as Senior Director Teva UK Limited in 2006, following the acquisition of IVAX Pharmaceuticals UK. Prior to joining Teva, he served three years at IVAX Pharmaceuticals UK as Director of Generics. Mr. Daniell received his BSc in chemistry from the University of Auckland.
Sven Dethlefs has been serving as Global Head of Respiratory Medicines, Global Specialty Medicines since January 2016. Prior to that, he had served as Chief Operating Officer, Teva Global Operations, since October 2013. Mr. Dethlefs joined Teva as General Manager, Teva Germany in 2008. Prior to joining Teva, he served for over 11 years as a partner at McKinsey & Company. Mr. Dethlefs received his PhD in biochemistry from the FU Berlin/Pasteur Institute Paris.
About Teva
Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) is a leading global pharmaceutical company that delivers high-quality, patient-centric healthcare solutions used by approximately 200 million patients in over 60 markets every day. Headquartered in Israel, Teva is the world’s largest generic medicines producer, leveraging its portfolio of more than 1,800 molecules to produce a wide range of generic products in nearly every therapeutic area. In specialty medicines, Teva has the world-leading innovative treatment for multiple sclerosis as well as late-stage development programs for other disorders of the central nervous system, including movement disorders, migraine, pain and neurodegenerative conditions, as well as a broad portfolio of respiratory products. Teva is leveraging its generics and specialty capabilities in order to seek new ways of addressing unmet patient needs by combining drug development with devices, services and technologies. Teva's net revenues in 2016 were $21.9 billion. For more information, visit www.tevapharm.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are based on management’s current beliefs and expectations and are subject to substantial risks and uncertainties, both known and unknown, that could cause our future results, performance or achievements to differ significantly from that expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to:
- uncertainties relating to the potential benefits and success of our new structure and recent senior management changes as well as the potential success and our ability to effectively execute a restructuring plan;
- our generics medicines business, including: that we are substantially more dependent on this business, with its significant attendant risks, following our acquisition of Allergan plc’s worldwide generic pharmaceuticals business (“Actavis Generics”); our ability to realize the anticipated benefits of the acquisition (and any delay in realizing those benefits) or difficulties in integrating Actavis Generics; the increase in the number of competitors targeting generic opportunities and seeking U.S. market exclusivity for generic versions of significant products; price erosion relating to our generic products, both from competing products and as a result of increased governmental pricing pressures; and our ability to take advantage of high-value biosimilar opportunities;
- our specialty medicines business, including: competition for our specialty products, especially Copaxone®, our leading medicine, which faces competition from existing and potential additional generic versions and orally-administered alternatives; our ability to achieve expected results from investments in our product pipeline; competition from companies with greater resources and capabilities; and the effectiveness of our patents and other measures to protect our intellectual property rights;
- our substantially increased indebtedness and significantly decreased cash on hand, which may limit our ability to incur additional indebtedness, engage in additional transactions or make new investments, and may result in a downgrade of our credit ratings;
- our business and operations in general, including: our ability to develop and commercialize additional pharmaceutical products; manufacturing or quality control problems, which may damage our reputation for quality production and require costly remediation; interruptions in our supply chain; disruptions of our or third party information technology systems or breaches of our data security; the failure to recruit or retain key personnel;the restructuring of our manufacturing network, including potential related labor unrest; the impact of continuing consolidation of our distributors and customers; variations in patent laws that may adversely affect our ability to manufacture our products; our ability to consummate dispositions on terms acceptable to us; adverse effects of political or economic instability, major hostilities or terrorism on our significant worldwide operations; and our ability to successfully bid for suitable acquisition targets or licensing opportunities, or to consummate and integrate acquisitions;
- compliance, regulatory and litigation matters, including: costs and delays resulting from the extensive governmental regulation to which we are subject; the effects of reforms in healthcare regulation and reductions in pharmaceutical pricing, reimbursement and coverage; potential additional adverse consequences following our resolution with the U.S. government of our FCPA investigation; governmental investigations into sales and marketing practices; potential liability for sales of generic products prior to a final resolution of outstanding patent litigation; product liability claims; increased government scrutiny of our patent settlement agreements; failure to comply with complex Medicare and Medicaid reporting and payment obligations; and environmental risks;
- other financial and economic risks, including: our exposure to currency fluctuations and restrictions as well as credit risks; the significant increase in our intangible assets, which may result in additional substantial impairment charges; potentially significant increases in tax liabilities; and the effect on our overall effective tax rate of the termination or expiration of governmental programs or tax benefits, or of a change in our business;
and other factors discussed in our Annual Report on Form 20-F for the year ended December 31, 2016 (“Annual Report”), including in the section captioned “Risk Factors,” and in our other filings with the U.S. Securities and Exchange Commission, which are available at www.sec.gov and www.tevapharm.com. Forward-looking statements speak only as of the date on which they are made, and we assume no obligation to update or revise any forward-looking statements or other information contained herein, whether as a result of new information, future events or otherwise. You are cautioned not to put undue reliance on these forward-looking statements.