TEL AVIV--(BUSINESS WIRE)--Aspect Imaging (www.aspectimaging.com), the developer of compact high-performance MRI systems, will present the benefits of MRI inside the NICU and will demonstrate the workflow of the Embrace® Neonatal MRI System at RSNA 2017 in Chicago, Booth #2771. Embrace® Neonatal MRI System enables preparation and scanning of newborns in less than an hour, without having to transport them from the Neonatal Intensive Care Unit (NICU). To schedule a live demonstration at RSNA, please contact Dr. Andrew Lonergan VP sales and Marketing of Aspect Imaging.
Additionally, Aspect Imaging and Natus Medical Inc, a leading provider of healthcare products and services, are collaborating to exclusively distribute Embrace® Neonatal MRI system in North America. With this partnership, Embrace® will reach more infants in NICUs in the US and Canada and improve neonatal care.
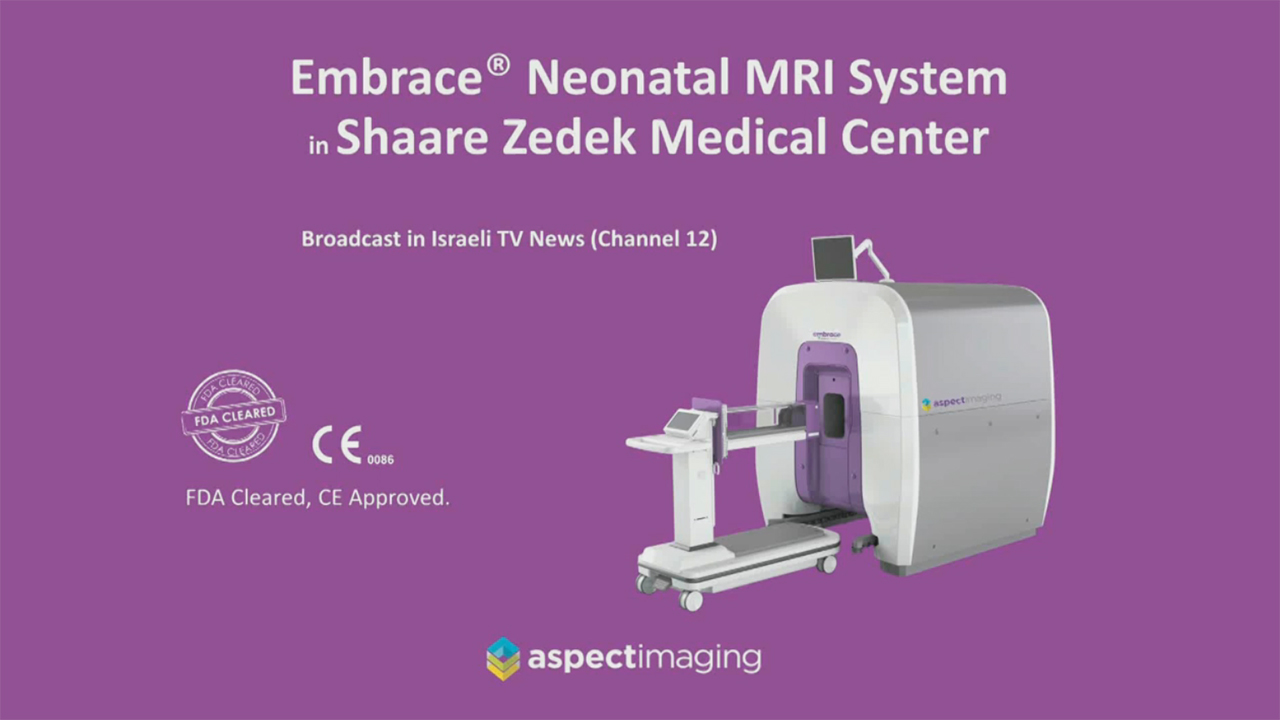
See videos below showing two different babies being scanned at Shaare
Zedek Medical Center in Jerusalem with the Embrace® MRI system:
https://youtu.be/LXFOXYp2-DY
(Channel 2 News, Israel)
https://vimeo.com/242058650
(Typical workflow)
Embrace® Neonatal MRI System does not require a special safety zone or an RF-shielded room, therefore, the system can be placed inside the NICU. Since the system is fully enclosed, medical device implants in close proximity (outside the magnet bore) are not required to be ‘MR Conditional’ or ‘MR Safe’.
Embrace® is acoustically quieter during scanning, compared to traditional whole-body scanners, and has a permanent magnet that is always active and thus requires no electrical, cryogenic or water cooling. Embrace® has an integrated, temperature-controlled incubator-like patient bed, which enables continuous control of the infant’s environmental temperature, allowing continuous monitoring of its vital parameters.
“We’re excited to unveil the Embrace® Neonatal MRI System in the US at RSNA 2017, where we can show our new approach to “MRI inside the NICUTM”. Our vision is to help premature babies, one of the most vulnerable populations in the world, with our technology and to improve and get a better chance to become healthy adults,” said Uri Rapoport, CEO and Founder of Aspect Imaging.
About Aspect Imaging
Aspect Imaging (www.aspectimaging.com) is a world leader in the design and development of complete, compact MRI and NMR systems using permanent magnet at 1 and 1.5 Tesla. Our unique technology platform is the basis for a wide range of products, spanning preclinical, medical, oil and gas, and advanced industrial markets. In the medical market, Aspect Imaging has several medical programs underway, including Embrace® and WristViewTM which are in full production, and a dedicated Stroke MRI for the ER which is under development. In the preclinical research market, the M-series compact MRI delivers a wide variety of in vivo and ex vivo applications. Advanced industrial applications include rheological analysis of both food products and industrial drilling mud with Aspect Imaging’s Flowscan. The AI-60 NMR is used for real-time, continuous flow-through stream analysis of dense and opaque materials such as crude oil, HF, and silicon production.
Leadership
Uri Rapoport, CEO & Founder