LOUISVILLE, Ky.--(BUSINESS WIRE)--There are now two reasons for patients to flinch at the touch of a stethoscope: because it’s cold, of course; and because it’s covered with germs.
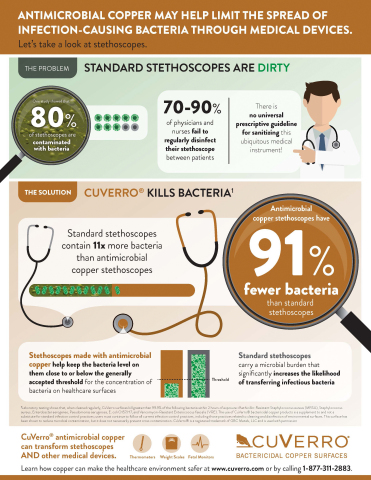
A new study1 from medical researchers reveals that 80 percent of the stethoscopes they studied were contaminated by high concentrations of bacteria, so much so that they present as much risk of transferring infectious bacteria as the hands of physicians after a physical exam.
The study, published by American Journal of Infection Control, compared bacteria loads on today’s standard stethoscopes and scopes fabricated with antimicrobial copper alloys that continuously kill 99.9 percent of bacteria. CuVerro® is a leading producer of EPA-registered antimicrobial copper.
Researchers discovered that stethoscopes made with traditional materials on the chest pieces, tubing, and diaphragms expose patients to an average of 127.1 CFUs (colony forming units of bacteria). In contrast, the stethoscopes made with antimicrobial copper exposed patients to an average of only 11.7 CFUs. The copper-alloy devices carried bacteria burdens 91 percent lower than standard scopes.
This difference could be significant in an age where patients and medical professionals are increasingly concerned about the incidence of transmitting bacteria that cause healthcare-associated infections, a problem that results in 99,000 patient deaths each year in US hospitals and leads to annual direct and indirect costs totaling between $96 and $147 billion.2
The study, led by Dr. Michael G. Schmidt, Vice Chairman of Microbiology and Immunology at the Medical University of South Carolina, pointed to evidence that there is only a one-in-ten chance that health professionals routinely disinfect their stethoscopes, making the effects of incorporating copper into the design of stethoscopes even more compelling.
The report noted there isn’t a “consistently applied routine of cleaning and disinfecting stethoscopes between patient encounters” even though they come in frequent contact with the "unsanitized skin of patients and the hands, face, neck and clothing of health care workers.”
“Based on these trial results,” Dr. Schmidt noted, “we can say that adding antimicrobial copper stethoscopes to a healthcare facility’s bundle of infection control measures would likely help to limit the spread of infectious agents. In other studies, antimicrobial copper touch surfaces have been demonstrated to work in concert with existing hygiene procedures to help create safer environments.”
The stethoscope study was conducted variously at the Medical University of South Carolina’s Children’s Hospital, the Department of Emergency Medicine at the University of New Mexico, and North Shore University Hospital in New York.
About CuVerro®
CuVerro is manufactured by GBC Metals, LLC, doing business as Olin Brass, a wholly owned subsidiary of Global Brass and Copper, Inc. which is a subsidiary of Global Brass and Copper Holdings, Inc. (NYSE:BRSS), the leading manufacturer and distributor of copper, copper‐alloy and bactericidal copper sheet, strip, plate, foil, rod, ingot and fabricated components in North America and one of the largest in the world. GBC Metals engages in the melting, casting, rolling, drawing, extruding and stamping of specialized copper and copper alloys finished products from scrap, cathode and other refined metals. (OB‐0034‐1510)
1 American Journal of Infection Control
2 Centers for Disease Control and Prevention