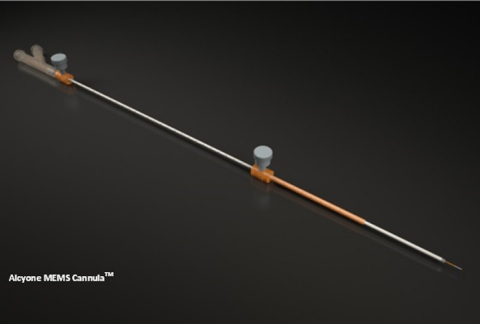
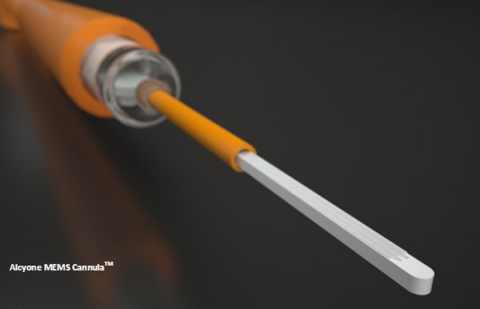
LOWELL, Mass.--(BUSINESS WIRE)--Alcyone Lifesciences, Inc., a leader in neural intervention systems for neurological conditions, announced that the Alcyone MEMS Cannula (AMC™), a neuro-ventricular cannula, has received FDA clearance. The AMC is a dual-lumen, MRI-compatible injection and aspiration cannula for use in the brain. The AMC is not intended for implant, and it is intended for single patient use only.
"I am thrilled for the Alcyone team. They are a highly talented and hard working group that has achieved this important company milestone in an efficient and skilled way,” commented Alcyone Lifesciences’ Board member Richard Upton of Harbor Light Capital Partners. “I look forward to helping them develop additional products and, ultimately, providing the best solutions for patients and their families."
“The neuroscience community is pioneering new therapeutic agents including gene therapy, antibody and oncolytic biologic therapy that hold great promise in treating chronic CNS disorders, but unfortunately they have lacked a clinically effective technology for precise CNS delivery direct to a neurological target and for optimal bio-distribution,” said PJ Anand, Founder & CEO of Alcyone Lifesciences. “Given that the very potential of these new agents is dependent on optimal bio-distribution, it is our hope that the AMC will offer a solution for this critical unmet clinical need and further open the gates to novel therapeutic options for patients.”
“The AMC was developed using our game-changing proprietary microelectromechanical system (MEMS) platform. Without burdening the neurosurgery community with unnecessary additional capital equipment, the AMC can be utilized with any existing commercial imaging and stereotactic system. Neurosurgeons can select a target, navigate the AMC precisely to the target, and observe in real-time the precision delivery of the therapeutic agent, all under intra-procedural MRI guidance”, said Deep Singh, Director of Product Development at Alcyone Lifesciences. “In addition to the MEMS tip which has dual micro-channels, the AMC features a unique patented distal end design that helps prevent reflux or back flow along the cannula shaft, which can be a significant drawback with current devices. The AMC platform device is designed for optimal targeted bio-distribution and neurosurgeon’s ease of use”.
About Alcyone Lifesciences, Inc.
Alcyone Lifesciences, based in Lowell, Massachusetts, is a privately-held medical device company focused on development of novel treatment modalities for chronic neurological conditions. The Company's patented technology platform is based on a uniquely engineered amalgamation of microfabrication technologies along with advanced biomedical engineering with core product focus on targeted drug therapy and hydrocephalus. Alcyone's team of scientists, physicians and advisers includes recognized leaders in the field of neurology and neurosurgery. For more information, please visit www.alcyonels.com