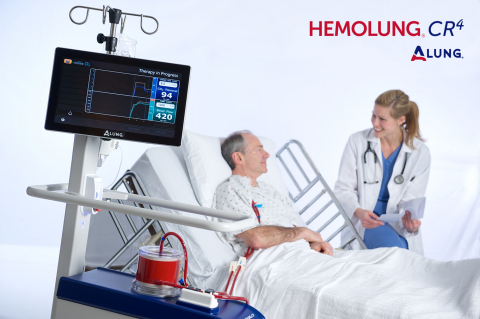
PITTSBURGH--(BUSINESS WIRE)--ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, today announced the limited launch of its next generation Hemolung CR4 Controller. The Hemolung CR4 Controller is part of the Hemolung Respiratory Assist System (RAS) which provides minimally invasive extracorporeal CO2 removal as an alternative or supplement to mechanical ventilation for patients suffering from acute respiratory failure.
“The development of the Hemolung CR4 Controller represents our commitment to making ECCO2R systems that are safe, simple and effective,” noted Peter DeComo, ALung Chairman and CEO. “In designing this device, we worked extensively with ICU nurses to understand their needs and workflow. The result of this effort is a device that retains all of the best features of our first generation Hemolung CR3 Controller, while being even simpler to operate. In the coming months, we will pair this new controller with an updated Hemolung Cartridge Kit featuring a fully pre-connected circuit to further simplify the delivery of Hemolung therapy.”
The Hemolung CR4 Controller is built on a modern platform with significant hardware and software upgrades. The most notable feature of the new controller is a vibrant touchscreen user interface which is completely intuitive and provides the right information at the right time. The system continues to provide the most intelligent monitoring of any extracorporeal gas exchange device, including the measurement of actual extracorporeal CO2 removal, a technique pioneered by ALung.
Other features of the device bring improved usability, safety, and reliability. Notably, these include:
- A smaller device which can be more easily positioned near the patient’s bed.
- A 360 degree handle and larger casters for easy maneuvering of the system, and facilitation of patient ambulation, from any position.
- Multidirectional routing of tubing, allowing the device to be placed on either side of the bed.
- A new quad-head vacuum pump, which generates sweep gas flow with significantly less noise.
- An improved oxygen circuit for increased safety and reliability.
- Improved accessibility of all gas tubing connections which have been moved from the back to the side of the device.
ALung will showcase the Hemolung CR4 Controller at the International Symposium of Intensive Care and Emergency Medicine (ISICEM) in Brussels, Belgium on March 17-20, 2015. The device has been launched on a limited basis to reference centers that will evaluate the product in clinical use. It is expected to be released to select markets in the second half of 2015.
About ALung Technologies
ALung Technologies, Inc. is a privately-held Pittsburgh-based developer and manufacturer of innovative lung assist devices. Founded in 1997 as a spin-out of the University of Pittsburgh, ALung has developed the Hemolung RAS as a dialysis-like alternative or supplement to mechanical ventilation. ALung is backed by individual investors and venture firms including Allos Ventures, Birchmere Ventures and West Capital Advisors, LLC.
For more information about ALung and the Hemolung RAS, visit www.alung.com.
This press release may contain forward-looking statements, which, if not based on historical facts, involve current assumptions and forecasts as well risks and uncertainties. Our actual results may differ materially from the results or events stated in the forward-looking statements, including, but not limited to, certain events not within the Company’s control. Events that could cause results to differ include failure to meet ongoing developmental and manufacturing timelines, changing GMP requirements, the need for additional capital requirements, risks associated with regulatory approval processes, adverse changes to reimbursement for the Company’s products/services, and delays with respect to market acceptance of new products/services and technologies. Other risks may be detailed from time to time, but the Company does not attempt to revise or update its forward-looking statements even if future experience or changes make it evident that any projected events or results expressed or implied therein will not be realized.